Introduction
Peripheral Nerve Injury (PNI) is occurred relatively commonly
in the clinic, which often leads to sensory and motor dysfunction
of limbs, and brings serious burden to family and society. Clinical
and academic efforts are focusing on how to improve PNI repair.
Schwann cells support nerve regeneration and motor function recovery by secreting a range of neurotrophic factors, cleaning damaged axons and myelin, and providing structural guidance, according to several studies [1,2], for these reasons, these cells are an
ideal therapeutic target for future clinical strategies.
Under pathological conditions, SCs regain the function of promoting peripheral nerve regeneration and repair [3-5]. To understand the role of SCs in repair of PNI, it is important to understand
the injury response during Wallerian degeneration. Through this
process, SCs will become de-differentiated, similar to newborn
SC progenitor cells, and will take on the identity of repair SCs [3,
6,7], which will proliferate and migrate to the wounded site [8].
There they aid in the creation of a permissive microenvironment
for axon renewal and nerve regeneration [9-14]. Considering the
critical roles played by SCs, treatments that might speed up the
proliferation and migration of SCs could aid in the repair and regeneration of PNI.
Previous studies have shown that Mesenchymal Stem Cell
(MSC) implanted in vivo or cocultured with peripheral nerve
extracts from the damaged sciatic nerve In vitro, can differentiate into SCs phenotype, providing necessary support and nourishment for axonal regeneration [15-18]. However, according to
Sowa’s research [19], transplanted MSCs, on the other hand, significantly enhance axonal outgrowth, myelin production, and the
repair of denervated muscle atrophy, nevertheless, do not differentiate into SCs. This suggests that the therapeutic effect of transplanted MSCs is due to the indirect regeneration of endogenous
SCs via a cellular paracrine mechanism rather than trans differentiation. Therefore, research into the effect of MSCs on SCs, particularly their proliferation and migration, is extremely important
for therapeutic purposes.
As we known, bone marrow, as a systemic cell bank, contains
various cell types, including MSC, macrophages, vascular endothelial cells and fibroblasts, here, be called bone marrow-derived
cells (BMDCs), which has been proved to offer several regenerative benefits for tissue and organ injuries [20-23] also, for peripheral nerve injury [24-26]. However, the mechanism of BMDCs promoting nerve regeneration, by SCs or other means, is remain unknown, further more elevating affection of BMDCs on the proliferation and migration of SCs is not report. Therefore, in this study,
we aim to understand the influence of BMDCs on the proliferation
and migration SCs, providing a potential new method for repair of
injured peripheral nerves in clinical.
Materials and methods
Animals
Shanghai SLAC Laboratory Animal Co., Ltd., China, provided
36 healthy male and female mature (6–8 weeks) C57Bl/6 mice
weighing 22–26 g (license No. SYXK (Hu) 2012-0001). All mice
were kept in the Central Laboratory of Bengbu Medical College in
China, where they were kept in a 12-hour light/dark cycle at 22°C
with a humidity of 40–67% and were free of particular pathogens.
All operations were carried out in line with the National Institutes
of Health Guide for Care and Use of Laboratory Animals and the
Ministry of Science and Technology of China’s Guidance Suggestions for the Care and Use of Laboratory Animals (2006). The Institutional Review Committee of China’s Bengbu Medical College
gave its approval to the animal trials.
By administering sodium pentobarbital (40 mg/kg) intraperitoneally, all mice were rendered completely unconscious, and then
the proceed was doing as follow [27].
Isolation of mouse BMDCs
BMDCs were extracted from the bone marrow of the femur
and tibia. The bone marrow was extracted in Dulbecco’s Modified
Eagle’s Medium (DMEM; Hyclone, Logan, UT, USA) containing 10%
fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin
after being lysed with erythrocyte lysis buffer. After that, homogeneous cell suspensions were saved for co-culture with sciatic
nerve segments.
Sciatic nerve tissue harvest
Sciatic nerve tissue was harvested according to an our previously reported methods [27]. In this experiment, the sciatic nerve was cut into 5 mm length segments, which will co-culture with
BMDCs.
In vitro culture
In group 1, the nerve segments were placed in a 6-well plate
with DMEM, 10% FBS, and 1% penicillin/streptomycin as the only
growth media. In group 2, BMDC suspensions were seeded on a
6-well plate first, and then nerve segments were inserted directly
in the plate for co-culturing with BMDCs. Every two days, the medium was replaced.
Separation and culture of SCs
SCs were isolated from cultured nerve segments. On 3, 7 days
after In vitro culture, the nerve segments were rinsed with PBS
and cut into smaller pieces. Then, the pieces were digested in dissociating enzyme solution which was prepared by dissolving collagenase NB4 (Serva, Heidelberg, Germany) in DMEM at a concentration of 0.2% (0.27 U/mL) at 37◦C, 5% CO2 for 60 min, followed
by 0.25% trypsin (Gibco) for 10 min. The mixture was centrifuged
at 600 × g for 10 minutes. After removing the supernatant, the cell
was re-suspended in SC culture medium consisting of DMEM supplemented with 10% FBS, 2 μM forskolin (Sigma, St. Louis, MO,
USA), 10 ng/mL heregulin-β-1 (PeproTech, Rocky Hill, NJ, USA),
and 50 ng/mL basic fibroblast growth factor (PeproTech). The cell
suspension was seeded in a flask at and incubated at 37°C, 5%
CO2, and allowed to adhere overnight.
Western Blot
The proteins extracted from SCs using Radioimmunoprecipitation assay (RIPA) buffer were separated by SDS-PAGE, and then
the proteins were transferred to polyvinylidene fluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). The membrane was
incubated with anti-P75NTRprimary antibodies overnight, 4oC.After
washing with TBST, the PVDF membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies,
and the immune complexes were visualized using Pierce™ ECL
Western Blotting Substrate (Thermo Fisher).
SCs Proliferation Assay
Prepared SCs which obtained from nerve segments on3, 7days
after
In vitro culturing in two groups were seeded into 96-well
plates at a density of 1 × 10
4 cells/well. MTT solution (Solarbio)
was added into each well, followed by a 4h incubation. The ab-
sorbance, or optical density (OD), was measured at 570 nm with a
microplate reader (TECAN).
Brdu stain
Prepared SCs were seeded into 24 well plates at a density of 5 ×
10
4 cells/ml, and then grown for 48 H in SC culture medium. After
48 h, the SC culture medium was replaced with fresh SC culture
medium and 10 μM of BrdU, and the culture was then incubated
at 37°C for 24 h. The following day, SCs were fxed with 4% PFA,
and then were incubated with 1N HCl on ice and then with 2N
HCl at room temperature for DNA hydrolysis. To detect incorporated BrdU, samples were stained with BrdU (1:500; Thermo). The
number of BrdU+ SCs was calculated from multiple fields of view
under the microscope using Image (n=5, five random regions).
Tanswell experiment
Prepared SCs which obtained from nerve segments on 3,7 days
after In vitro culturing in each group were suspended in serum-free medium and the cell density was adjusted to 5x104 cells/
mL. 24-well plates were used and 500 ul SC culture medium were
added into each well, then the transwell chamber also were put
into well, 200 ul SC suspensions were added into upper chamber
and cultured for 48 h. Then the transwell chamber were taken out
and washed with PBS. The cells in the upper chamber were wiped
with a cotton swab, washed with PBS, fixed with 4% PFA for 20
min, stained with 0.5% crystal violet for 5 min, and washed with
PBS. The number of migrated cells attached to the lower surface
of the chamber was observed under a microscope, and 5 fields of
view were randomly selected and averaged.
The Migration of SCs from nerve segment
On 3,7 days after In vitro culture, the nerve segments in each
group was observed by phase contrast microscopy (Olympus, Tokyo, Japan) at 100× magnification, and then the distance of SCs
migration were record and compared(5 fields of view were randomly selected and averaged).
SCs co-cultured with PLA filament
Prepared SCs were seeded at a density of 5 × 104cells/ml in
SC culture medium into 6-well plates, and then sterilized PLA filament were add in for co-culturing. After incubated at 37°C, 5%
CO2 for 48 h, under a microscope, SCs and PLA filament was observed, and which morphology and arrangement was described.
Statistical analysis
All data is presented as a mean standard deviation. The values
were subjected to a two-sample t-test, and the least significant
difference test using SPSS 22.0 software (SPSS, Chicago, IL, USA)
for quantitative comparison and analysis. The threshold for statistical significance was fixed at P 0.05.
Results
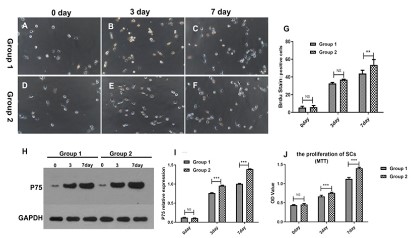
BMDCs enhanced the proliferation of SCs
MTT assay and Brdu stain was used to detect the activity of
Schwann cells in each group. Compared with group 1, BMDCs in
group 2 induced a robustly higher proliferation rate at the 3rd, 7th
day, indicating by MTT assay and which were statistically significant (P<0.05). Also, the Brdu positive SCs significantly increased in
group 2 at the 7th day, but not at the 3rd day.
P75NTR, was a low-affinity receptor for multiple neurotrophins, was expressed in developing SCs, was involved in the proliferation and anti-apoptotic of SCs. So, we detect the difference of
p75NTR expression between two groups. Compared with group 1,
the expression of P75NTR in group 2 was significantly increased at
the 3rd, 7th days (P<.05) (Figure 1).
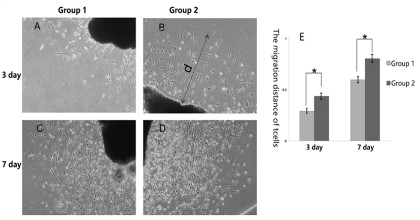
BMDCs promoted the migration of SCs
Since the success of nerve repair is highly dependent on the
ability of SCs to effectively migrate across the injury site, how to
improving the migration of SCs is popular topic. In our experiment, SCs migrated from the nerve segments when cultured in
vitro at the 3rd day, as time goes on, at the 7th day, a lot of SCs migrated, expanded and connected into sheets in all groups, observed by phase contrast microscopy. In addition, compared with
group 1 (nerve segments cultured alone), the migration distance
of SCs in group 2 (BMDCs co-cultured with never segments), was
significantly longer at the 3rd and 7th day, and which were statistically significant (Figure 2).
Also, Transwell assay was used to detect the migration of SCs in
each group. Compared with group 1, the migration of SCs in group
2 was significantly increased at the 7th day and were statistically
significant (P<0.05). It can be seen, BMDCs promoted the migration of SCs (Figure 3).
BMDCs promoted the SCs forming Bungner’s brand-liked
structure
In Wallerian degeneration, SCs form aligned elongated tubular structures called bands of Bungner that provide guidance to
regrowing axons and support their linear regeneration. So, we
detected SCs whether forming Bungner’s brand-liked structure in
vitro. In our experiment, when SCs obtained from group 2 were
co-cultured with filament like structure made of PLA, the migration of SCs was orderly and arranged along the filament forming
Bungner’s brand-liked structure, however, in group 1, SCs were
disorderly and not arranged along the filament (Figure 4).
Discussion
SCs are the unique glial cells in the peripheral nerve [28]. In the
event of a peripheral nerve injury, SCs change their morphology,
function, and play a new role as repair cells [3,6,7]. Through this
process, a dynamic SCs reprogramming maybe briefly divide into
two stages. At the early stage, SCs go through dedifferentiation,
proliferation, myelin sheath clearance, this stage is characterized
by proliferation of SCs [14,29]. Then at the later stage, repair
SCs migrate into the lesion site, form bunger’s band, which will
create a permissive environment for nerve regeneration [30,31].
The change of SCs in two stages regulate by various cellular
and molecular factors [14,32-34]. Factors that enhance SCs
proliferation and promote SCs migration after peripheral nerve injury will benefit nerve regeneration and functional recovery.
In the current study, we examined the modulation of BMDCs
for proliferation and migration of SCs by using western blot, cell
proliferation assay, cell migration assay. In our results, BMDCs
accelerate the proliferation of SCs, and the significant tendency
was beginning on the 3rd day after BMDCs co-cultured with nerve
segments, was peaking on the 7th day. Also, BMDCs promote the
migration of SCs, nevertheless, the significant effectiveness was
detected on the 7th, which was justly consistent with the character
of SCs in Wallerian degeneration. We also find the migration of SCs
was orderly and arranged along the filament forming bungner’s
brand-liked structure when co-cultured with filament.
Previous study has indicated numerous methods that could
promote the proliferation and migration of SCs [35-37]. However,
these methods frequently involve in complex, difficult to be
popularized, and what is more, these factors cannot enhance SC
proliferation in the early stage by one hand and further promote
SC migration in the late stage by the other hand. In our research,
we take BMDCs co-cultured with nerve segments, which is a
simple, easy to implement, and both enhancing the proliferation
in early stage and promoting the migration in late stage method,
in addition, compared with previous reports, our method have
other superiority, which can be list as follow:①As we prior study,
taking BMDCs co-cultured with nerve segments is an effective way
to generated a large of repair SCs in a short time frame, which is of
the essence for clinical purposes. ②Based on “BMDCs promoting
the proliferation and migration of SCs”, when combination of
BMDCs and SCs transplanted in artificial nerve conduits for nerve
repair, the BMDCs will enhance the proliferation and sustain the
survival of SCs in conduit site, and sustaining the survival of SCs
was considered as a key factor for successfully repair in the fields
of tissue engineering.
In recent studies, the importance of micro environment at the
nerve lesion was recognized [10,38]. The dynamic biochemical
changes in the micro environment ultimately improved the
proliferation and migration of SCs. After nerve injury, beside SCs
activated, other cell types contain macrophages, neutrophils,
fibroblasts and endothelial cells, are also recruit and active
at the site of lesion, all kinds of cells secrete various growth
factors, cytokines, interleukins, ECM, and form a dynamic micro
environment, in turn, these environmental cues promote the
proliferation and migration of SCs by means of affecting multiple
intracellular signaling pathways [10,34,39]. BMDCs are a mixed
population of cells, including stem cells, mesenchymal cells, blood
system cells, fibroblasts and vascular endothelial cells, and so on.
In our trail, we take these cells co-cultured with nerve segments
In vitro, and find that these cells infiltration in the inner of nerve
segments, which is mimics the dynamic change of the micro
environment in the site of nerve lesion in vivo and at last lead the
proliferation and migration of SCs, we guess.
Taken together, this study provides a new method to improve
the proliferation and migration of SCs In vitro and imply the
potential role of the BMDCs and SCs in the treatment of peripheral
nerve injury. However, there are still have some problems in our
researcher that have not been solved, such as, further clarifying
the mechanism and verifying the effect that combination of BMDCs
and SCs transplanted in artificial nerve conduits for nerve repair.
Declarations
Ethics approval and consent to participate: All of the animal
experiments in this study were performed in accordance with
the National Institutes of Health Guide for Care and Use of
Laboratory Animals, the Ministry of Science and Technology of
China’s Guidance Suggestions for the Care and Use of Laboratory
Animals (2006), and were approved by the laboratory animal
ethical committee of Bengbu Medical College (Number: BYYFY-
2021KY18). All methods are reported in accordance with ARRIVE
guidelines for the reporting of animal experiments.
Consent to publish: Not applicable.
Availability of data and materials: The datasets used and/
or analysed during the current study are available from the
corresponding author on reasonable request.
Competing interests: The authors declare that they have no
competing interests.
Funding: This research support by the Key project of Natural
Science Foundation of Bengbu Medical College (No. BYKY1837ZD
and 2020byzd111); the Key University Natural Science Research
Project of Anhui Province of China (No. KJ2021A0723). The
funders had no role in study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
Authors’ contribution: Xiaopan Wang designed the study,
conducted the experiments, analyzed the data, obtained the
funding and wrote the paper. Min Wu obtained the funding and
provided the critical revision of the paper. Jun Yan designed the
study and provided the critical revision of the paper. Peishuai
Zhao and Xiaotian Chen participated in experiments. All authors
approved the final version of the paper.
Acknowledgements: Not applicable.
References
- Liu Y, Zhang Z, Qin Y, et al. A new method for Schwann-like cell
differentiation of adipose derived stem cells. Neurosci Lett. 2013;
551: 79-83.
- Zhang R, Rosen JM. The role of undifferentiated adipose-derived
stem cells in peripheral nerve repair. Neural Regen Res. 2018; 13:
757-763.
- Jessen KR, Mirsky R. The Success and Failure of the Schwann Cell
Response to Nerve Injury. Front Cell Neurosci. 2019; 13: 33.
- Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved
in peripheral nerve repair after injury. Cell Mol Life Sci. 2020; 77:
3977-3989.
- Stassart RM, Woodhoo A. Axo-glial interaction in the injured PNS.
Dev Neurobiol. 2021; 81: 490-506.
- Jessen KR, Mirsky R. Schwann Cell Precursors; Multipotent Glial
Cells in Embryonic Nerves. Front Mol Neurosci. 2019; 12: 69.
- Mirsky R, Woodhoo A, Parkinson DB, et al. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J Peripher Nerv Syst. 2008; 13: 122-135.
- Xiang FF YY, Tan XQ, Wei DQ, Yang K, Sun YL, et al. Effect of combination of acellular nerve grafts and stem cells for sciatic nerve
regeneration: A meta-analysis. Chinese Journal of Tissue Engineering Research. 2017; 21: 3602-3608.
- Bunge RP. Expanding roles for the Schwann cell: ensheathment,
myelination, trophism and regeneration. Curr Opin Neurobiol.
1993; 3: 805-809.
- Fornaro M, Marcus D, Rattin J, Goral J. Dynamic Environmental
Physical Cues Activate Mechanosensitive Responses in the Repair
Schwann Cell Phenotype. Cells. 2021; 10.
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors,
and peripheral nerve regeneration. Microsurgery. 1998; 18: 397-405.
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural
regeneration: basic insights. Brain Pathol. 1999; 9: 313-325.
- Zhang Z, Yu B, Gu Y, Zhou S, Qian T, et al. Fibroblast-derived tenascin-C promotes Schwann cell migration through beta1-integrin dependent pathway during peripheral nerve regeneration. Glia Mar.
2016; 64: 374-85.
- Jessen KR, Arthur-Farraj P. Repair Schwann cell update: Adaptive
reprogramming, EMT, and stemness in regenerating nerves. Glia
Mar. 2019; 67: 421-437.
- Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral
Nerve Regeneration: Current Options and Opportunities. Int J Mol
Sci. 2017; 18.
- Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral
Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int
J Mol Sci. 2016; 17.
- Wang H, Zhang H, Liu M, Wang N. Distal segment extracts of the
degenerated rat sciatic nerve induce bone marrow stromal cells to
express Schwann cell markers In vitro. Neurosci Lett. 2013; 544:
89-93.
- Zhang Z, Liu Y, Zhu X, Wei L, Zhu J, et al. Sciatic nerve leachate of
cattle causes neuronal differentiation of PC12 cells via ERK1/2 signaling pathway. J Vet Sci. 2018; 19: 512-518
- Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, et al. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo
without Differentiation into Schwann-Like Lineage. Plast Reconstr
Surg. 2016; 137: 318e-30e.
- Dou F, Wu B, Chen J, Liu T, Yu Z, et al. PPARalpha Targeting GDF11
Inhibits Vascular Endothelial Cell Senescence in an Atherosclerosis
Model. Oxid Med Cell Longev. 2021; 2021: 2045259.
- Huang Y, He B, Wang L, et al. Bone marrow mesenchymal stem
cell-derived exosomes promote rotator cuff tendon-bone healing
by promoting angiogenesis and regulating M1 macrophages in
rats. Stem Cell Res Ther. 2020; 11: 496
- Liu L, Guo S, Shi W, et al. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng Part A. 2021; 27: 962-976.
- Liu X, Zheng L, Zhou Y, Chen Y, Chen P, et al. BMSC Transplantation Aggravates Inflammation, Oxidative Stress, and Fibrosis and
Impairs Skeletal Muscle Regeneration. Front Physiol. 2019; 10: 87.
- Wang S, Zhu C, Zhang B, Hu J, Xu J, et al. BMSC-derived extracellular matrix better optimizes the microenvironment to support
nerve regeneration. Biomaterials. 2022; 280: 121251.
- Zhao J, Ding Y, He R, Huang K, Liu L, et al. Dose-effect relationship
and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell
Res Ther. 2020; 11: 360.
- Zhou LN, Wang JC, Zilundu PLM, Wang YQ, Ping Guo W, et al. A
comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration In vitro and in
vivo. Stem Cell Res Ther. 2020; 11:153.
- Wang XP, Wu M, Guan JZ, Wang ZD, Gao XB, et al. Pre-degenerated
peripheral nerves co-cultured with bone marrow-derived cells: a
new technique for harvesting high-purity Schwann cells. Neural
Regen Res. 2016; 11: 1653-1659.
- Huang G, Sun Z, Wu J, Shui S, Han X, et al. Calreticulin Promotes
Proliferation and Migration But Inhibits Apoptosis in Schwann
Cells. Med Sci Monit. 2016; 22: 4516-4522.
- Xu Z, Orkwis JA, DeVine BM, Harris GM. Extracellular matrix cues
modulate Schwann cell morphology, proliferation, and protein expression. J Tissue Eng Regen Med. 2020; 14: 229-242.
- Gomez-Sanchez JA, Pilch KS, van der Lans M, Fazal SV, Benito C,
et al. After Nerve Injury, Lineage Tracing Shows That Myelin and
Remak Schwann Cells Elongate Extensively and Branch to Form
Repair Schwann Cells, Which Shorten Radically on Remyelination.
J Neurosci. 2017; 37: 9086-9099.
- Wang Y, Zhang F, Zhang Y, Shan Q, Liu W, et al. Betacellulin regulates peripheral nerve regeneration by affecting Schwann cell migration and axon elongation. Mol Med. 2021; 27: 27
- Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms
Schwann cells of injured nerves to generate a repair cell essential
for regeneration. Neuron. 2012; 75: 633-47.
- Jacob C. Chromatin-remodeling enzymes in control of Schwann
cell development, maintenance and plasticity. Curr Opin Neurobiol. 2017; 47: 24-30.
- Tajdaran K, Chan K, Gordon T, Borschel GH. Matrices, scaffolds, and
carriers for protein and molecule delivery in peripheral nerve regeneration. Exp Neurol. 2019; 319: 112817.
- Chen Y, Fan Z, Dong Q. LncRNA SNHG16 promotes Schwann cell
proliferation and migration to repair sciatic nerve injury. Ann
Transl Med. 2021; 9: 1349.
- Cheng Z, Zhang Y, Tian Y, Chen Y, Ding F, et al. Cyr61 promotes
Schwann cell proliferation and migration via alphavbeta3 integrin.
BMC Mol Cell Biol. 2021; 22: 21.
- Zhou Z, Zhang N, Shi P, Xie J. Mechanism of miR-148b inhibiting cell
proliferation and migration of Schwann cells by regulating CALR.
Artif Cells Nanomed Biotechnol. 2019; 47: 1978-1983.
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev
Neurosci. 2007; 30: 209-233.
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, et
al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015; 162: 1127-39