Introduction
In Sri Lanka, OSCC is identified as the most common cancer
among males and 8th among females [1]. Chewing betel leaf with
smokeless tobacco and areca nut (BSTA) is shown to be the most
common reason for OSCC in the South and South-Eastern Asia
(SSEA) and the incidence in this region is much higher [2]. Therefore, these OSCCs are “betel quid induced (BQI)” and may behave
uniquely due to the changes in their Extracellular Matrix (EM).
The 5-year overall survival of OSCC patients who chewed areca
nut is shown to be significantly less than those who didn’t [3].
These OSCC patients have a 19.1 times higher chance of having a
coexisting oral potentially malignant conditions such as Oral Submucous Fibrosis (OSF) or leukoplakia with Oral Epithelial Dysplasia
(OED) [4]. Hence, their Pathological Excision Margins (PEM) have
a greater chance of being close or positive. Furthermore, wide
surgical excision to accommodate extensive lesions bordering
OED results in poor recovery and high morbidity. These patients
have field-cancerization which predispose to a higher chance of
local recurrences and the incidence of a second primary cancer
[3]. The effects of BSTA on the extracellular matrix and submucosal fibrosis may also affect the immediate post-surgical tissue
shrinkage. Mistry et al, in a study in a group of BQI OSCC patients
from India had 50% less immediate postoperative shrinkage in the
buccal mucosal cancers [5].
In addition, the fibrosis in the extra-cellular matrix caused by
BCTA, is believed to cause lymphatic occlusions, resulting in a decreased tumour metastasis to the neck. Sigh et al, showed 81%
negative necks in T4 patients with OSF, while it was 28.6% in patients without [6]. Chaturvedi et al and Siriwardena et al, both
found a reduce trend in metastasis to the neck in the background
of OSF [7, 8].
With the influence of the BQI EM, disease progression of OSCC
can be significantly different to OSCC without BQI EM. Therefore,
we aim to investigate the influence of pathological excision margins on recurrence and overall survival (3 and 5 year) of Sri Lankan
OSCC patients whose aetiology is BSTA.
Materials and methods
Histologically confirmed primary OSCC cases treated with surgery at Oral and Maxillofacial units of Sri Lanka for a period of 13
years were included. All patients were reviewed and monitored
closely during the first 5-years and annually thereon. The cases
that have lost to follow-up and the cases that have less than 3
years follow-up at the time of analysis, were excluded from the
study. All excisional biopsy specimens of selected cases were retrieved from the Department of Oral Pathology, Faculty of Dental
Sciences, University of Peradeniya. Demographic, clinical and histopathological data were gathered from patient’s clinic and hospital records. All margins taken for the purpose of surgical pathology report were re-assessed and distance from the main tumour
to mucosal margins (lateral margins) and to the deep margin were
recorded using the stage micrometer. Measurement was started
to record from 0.5 mm with 0.5 mm intervals (0 mm was recorded
as involved) up to 6.5 mm and beyond. Both lateral and deeper
PEMs were separately measured histologically and recorded. Both
margins were separately analyzed. Out of the two measurements
from each case, the closest measurement was recorded. For the purpose of statistical analysis readings were grouped as group A
(6.5 mm or more), B (5.5-6 mm), C (3.5-5mm), D (1.5-3 mm) and
E (1 mm or less-Involved).
Statistical analysis
Statistical analysis was performed using SPSS (Windows version 16) software. Data was analyzed using descriptive statistics.
Pearson’s Chi-square test was used to compare proportions. The
statistical significance was accepted at p < 0.05. Logistic regression was used to identify the relationship between margins with
recurrence and survival and Kaplan Meier survival curves were
generated to compare survival with different parameters.
Results
A total of 250 patients (Males: Females were 3:1) with follow-up details were included in the study. Total sample was grouped
into age categories and many patients were within 51-60 (31.6%)
and 61-70 (32%) age groups. There were 4 patients who were less
than 30 years of age. With reference to the site, OSCC of the buccal mucosa (BM) was predominant (54%) followed by the tongue
(19.2%). Fifty percent (50%) of the patients were stage IV, while,
stage 1 had the lowest cases with only 7.2%. From this study
group, all patients completed 3-years follow up while 186 had
5-years and above.
The most obvious factors influencing overall survival were,
the site of the primary and the stage of the disease. OSCC of the
tongue showed worse outcomes for both 3-year (p=0.002) and
5-year (p=0.022) overall survival compared to lower alveolar ridge
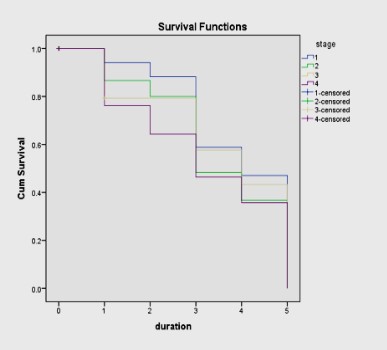
(LAR) and BM (Figure 1). Furthermore, a highly significant statistical correlation was identified between overall survival and the
stage of the disease for 3-yrs (p<0.001) and for 5-yrs (p=0.004)
(Figure 2). With advancing stage of the disease, the overall survival rate was significantly reduced irrespective of the site.
When considered the groups from A-E, although there is no
significant relationship with regards to 3-year survival and marginal clearance, there is a clear improvement of the overall survival. The same is clearly visible with 5-year overall survival. Furthermore, majority of local recurrences and regional recurrences
were within 3 years after the surgery. There were few cases of
local recurrences, nodal metastasis (regional recurrences) after 3
years (Table 1).
Table 2 describes the recurrences following radiotherapy.
There is no proper relationship with recurrence with post operative radiotherapy. The reason is the poor compliance of patients.
When all patients were categorized as clear margins (groups
A and B), close margins (groups C and D) and involved margins
(group E), a statistically significant association was found only between 3-year survival rate and the PEM status (p=0.029) (Figure 3)
and not with the 5-years the survival (p=0.114).
When the lateral margins and the deep margins were considered separately, the lateral margin clearance showed no significant benefit at 3 (p=0.186) or 5-year (p=0.421) survival. Deep
margin clearance was statistically correlated with a better survival
at 3-years (p= 0.005). However, the deep margin clearance was
not statistically significant for 5-year survival (p=0.067).
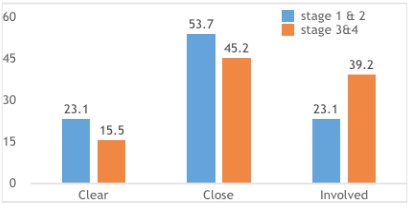
Majority of the sample included the close margin category
(48%) followed by <1 mm group (34%). The rest was with clear
margins (18%). Most patients present with late stage of the disease and the possibility of obtaining a clear margin was reduced
and most stage IV cases had PEMs <1 mm and the results were
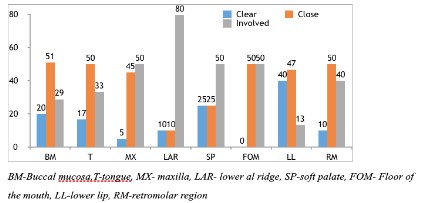
significant statistically (p=0.032) (Figure 4). The highest involved
excision margins were seen in patients with LAR cancer, whereas,
lower lip had the least (Figure 5). Many patients were treated
with surgery and 43.2% had post-operative radiotherapy. When
the treatment type and survival are considered, there was no significant association.
In order to identify the individual predictors for survival, forward and backward stepwise logistic regression was applied.
Variables used were age, sex, stage, lateral margin, deep margin
and treatment and when compared with survival, age, stage and
deep margin were identified as individual predictors for survival
(p=0.042, p<0.001, p<0.001) respectively. Logistic Regression procedure, requesting backward elimination of predictors was also
carried out in order to confirm the independent prognosticators
mentioned above (p=0.039, p=0.001, p=0.001).
Table 1: 3-year and 5-year survival and recurrences by margin status.
| Group |
Margin category |
3-year survival |
3-year recurrence (local) (regional) |
5-years survival percentage/proportions |
5-year recurrence (local) (regional) |
| Percentage/proportions |
| A |
>6.5 mm |
100% / (8/8) |
nil |
100% (5/5) |
nil |
| B |
5.5-6.5 mm |
59.46% / (22/37) |
(4/37) (5/37) |
41.67% / (10/24) |
(*1/24) (0/24) |
| C |
3.5-5 mm |
61.9% (52/84) |
(16/84) (7/84) |
44.07% (26/59) |
(**4/59) (0/59) |
| D |
1.5-3 mm |
58.36%, (21/36) |
(9/36) (2/36) |
40.7% (11/27) |
(**2/27) (0/27) |
| E |
<1 mm/0-1 mm |
48.24% (41/85) |
(44/85) (6/85) |
32.39% (23/71) |
(**4/71) (0/71) |
•Second primary **recurrences in between 3-5 years
Table 2: Recurrence after radiotherapy related to margins.
| Margin category |
RT+ |
RT- |
total |
RT+ rec + |
RT-rec + |
RT+rec + |
RT-rec+ |
| >6.5 mm |
2 |
5 |
7 |
0/2 (0%) |
0/5 (0%) |
3/16 (18.75%) |
5/31 (16.1%) |
| 5.5-6.5 mm |
14 |
26 |
40 |
3/14 (21.4%) |
5/26 (19.2%) |
|
| 3.5-5 mm |
41 |
43 |
84 |
16/41(39%) |
9/43 (20.9%) |
21/53 (39.6%) |
16/67 (23.8%) |
| 1.5-3 mm |
12 |
24 |
36 |
5/12 (41.6%) |
7/24 (29.1%) |
| <1 mm/0-1 mm |
39 |
44 |
83 |
18/41 (46.15%) |
23/42 (52.27%) |
18/41 (46.15%) |
23/42 (52.27%) |
RT radiotherapy, RT+ radiotherapy given, RT- radiotherapy not given, rec+ recurred, rec- not recurred
Discussion
Our study assessed the PEM of OSCC patients against 3-year
(250 patients) and 5-year (186 patients) survival. The main purpose was to assess the PEM status and its influence on the overall
survival of the patients with OSCC who chewed betel quid.
Habit of chewing BSTA is identified as the main aetiology for
OSCC in Sri Lanka and mostly in a the poor socio-economical
group 9. Our study agreed with the socio-demographic findings of
previous Sri Lankan cohorts in confirming that the BM predominate as the primary site, elderly males (50-70 years) are mostly
affected and most patients present to hospital at stage III and IV
of the disease 10.
The age and survival
Patients with OSCC over 60-years have poor general health and
they fare poorly than younger patients [11]. Even at a higher stage
of the disease, the younger patients have shown better survival
outcome than the older counterparts [12]. Warnakulasuriya et al,
in their study in the UK, showed a better prognosis among the
younger age of less than 45 years [13]. The findings were quite
different in the study by Garavello et al, where tongue cancer had
a higher mortality in patients younger than 40 years [14]. As BSTA
is the main causative agent in Sri Lanka, the usual age group is
over the age of 30 years [15]. In our cohort, the age group below
30 years were 1.6%, and the age group 41-50 were 10%, whereas
the age range of 51 to 70 years included close to 63%. Logistic
regression showed increasing ages as an independent risk factor
for both 3-year and 5-year survival.
The stage of the disease and survival
In our study, a clear reduction in survival was seen in patients
diagnosed as stages III and IV. Though, the stages III and IV of oral
cancer receive post-operative radiotherapy, their local control
and survival benefits remains very low, especially for stage IV. We
found that involved and close margins were mostly found in the
stages III and IV indicating the difficulty of complete excision of
such tumours and the added negative influence on survival. This
finding is supported by Roeland et al, in their study which showed
that stages I and II had a higher chance of being completely excised than stages III and IV (22.6% vs 5.1%) [16].
The primary site of cancer and survival
LAR had the highest incidence of involved margins (<1 mm)
(80%) followed by maxilla, soft palate and the floor of the mouth.
In contrast, lower lip had the least involved margins (13.3%) and
the highest percentage of clear margins (40%). Most studies report higher incidence of close margins with BM, tongue and mandibular alveolus. The site of the primary tumour influence prognosis due to the lympho-vascular supply which influence metastasis and the ability to achieve a clear excision margin. Tongue and
floor of the mouth (FOM) are the most common sites to have the
worse prognosis due to the anatomical location in close proximity
to the lymph nodes of the neck which encourage early metastasis.
In addition, most of the tongue and floor of the mouth cancers
are diagnosed late in contrast to the lip lesions, which in turn affects the prognosis of these patients [17]. Our study group had
the worst survival with tongue as the primary site followed by the
LAR and BM respectively. In contrast to this, oral cancer of the
BM is considered as a site of high loco-regional failure even in the
initial stages of the disease [18]. Five year disease specific survival
data from the Memorial Sloan-Kettering cancer centre ranks BM
at the sixth place in a descending order for survival, indicating the
significance of this site to others [19]. Shaw et al, points out that
squamous cell carcinoma of the BM presents at a late stage and
frequently have involved margins; thus the reason for the poor
prognosis. The authors further emphasize the fact that in SSEA
countries where habitual betel chewing which is the cause for
cancers of BM, has a better prognosis than similar lesions in the western countries that occur due to tobacco smoking and alcohol
[20].
The PEM and survival
PEM in patients with OSCC have a significant influence on survival. Though, most studies agree on a positive PEM to have a
significantly poor prognosis, McMahon et al, in their multicentre
study failed to implicate PEM as an independent predictor of survival and loco-regional control [21,22]. Barry et al, showed that
close PEM didn’t have an influence on recurrences or in survival
in patients with early stage tongue cancer [23]. Weijer et al, reported that, there was no significant increase in recurrences in
patients with close deep margins [24].
Interestingly, how the close PEM affects the overall prognosis
is unclear. Wong et al, found that close excision margins between
1mm to 1.6 mm did not necessarily increased the chances local
recurrences, but reduced the overall survival. They postulate that
the closeness of the tumour to the surgical margins correlate to
the tumour size and aggressiveness and has no direct correlation
[25]. They found a resection margin of less than 1.6 mm had a significantly higher reduction in 5-year survival and recommends a
close margin to be recognized as 1-2 mm. This cut-off margin has
been shown to vary with different studies. Chiou et al and Dik et
al claim that margins over 3mm to have the same survival benefit
of a margin over 5 mm and 6 mm respectively [26,27]. Similarly,
Zanoni et al and Nason et al, recommend margins over 2.2 mm
and 3 mm respectively. For aggressive disease where the surgery
is the main treatment modality, a more liberal PEMs of over 7
mm and 1 cm are suggested [28,29]. Due to this vast variation in
results, the best PEM is difficult to assess.
We studied a group of patients whose aetiology was predominantly betel chewing. Therefore, the influence of PEM on recurrences and survival of these patients are important as the changes
in the extracellular matrix may influence the biological behaviour
of the cancer.
We noted a clear reduction in 3-year and 5-year survival when
the PEM was involved (1 mm or less). When the deep PEMs were
categorized to involved (<1 mm), close (1.5-5 mm) and clear (5.5-6.5 mm), a statistical significance was found on 3-year survival but
not on 5-year survival. This result agrees with a meta-analysis by
Anderson et al, that showed a PEM of over 5 mm to have a better
prognosis regardless of the sub site of the oral cancer [30]. This
relationship was not noted with the lateral margins. The patient
who had PEM over 6 mm, all survived, though the sample size was
low to draw a statistically significant result.
Conclusion
A deep PEM of less than 1 mm clearly had a negative influence
on the overall 3-year and 5-year survival of our patients with betel
quid induced oral cancer. When deep PEMs were divided as close
(1.5-5 mm) and completely excised (over 5.5 mm), the 3-year
survival significantly improved. Therefore, attempting PEM over
5 mm would be recommended. In our study, the deep margin status had the most significant influence. Therefore, a 3-dimensional
tumour clearance is of outmost importance.
Declarations
Data availability statement: Apart from the data within the
text, raw data can be provided upon request.
Funding: None.
Conflict of interest disclosure: The authors have declared that
there is no conflict of interests.
Ethics approval statement: The study used archival data.
Patient consent statement: Not applicable.
Permission to reproduce material from other sources: Not
relevant.
Clinical trial registration: Not relevant.
References
- 144-sri-lanka-fact-sheets.pdf.
- Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid
chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer. 2014;
135: 1433-1443.
- Liao CT, Wallace CG, Lee LY, Hsueh C, Lin CY, et al. Clinical evidence
of field cancerization in patients with oral cavity cancer in a betel
quid chewing area. Oral Oncol. 2014; 50: 721-731.
- Merchant A, Husain SS, Hosain M, Fikree FF, Pitiphat W, Siddiqui
AR, et al. Paan with-out tobacco: an independent risk factor for
oral cancer. Int J Cancer. 2000; 86: 128-131.
- Mistry RC, Qureshi SS, Kumaran C. Post-resection mucosal margin
shrinkage in oral can-cer: quantification and significance. J Surg
Oncol. 2005; 91: 131-133.
- Singh G, Rana AS, Kumar A, Prajapati A, Kumar S, Singh P. Nodal
involvement in Oral Squamous Cell Carcinoma (SCC) patients with
and without Oral Sub Mucous Fibrosis (OSMF): A Comparative
Study. J Oral Biol Craniofac Res. 2017; 7: 171-177.
- Chaturvedi P, Vaishampayan SS, Nair S, Nair D, Agarwal JP, et al.
Oral squa-mous cell carcinoma arising in background of oral sub-mucous fibrosis: a clinicopathologi-cally distinct disease. Head
Neck. 2013; 35: 1404-1409.
- Siriwardena BSMS, Jayawardena KLTD, Senarath NH, Tilakaratne
WM. An Evaluation of Clinical and Histopathological Aspects of
Patients with Oral Submucous Fibrosis in the Background of Oral
Squamous Cell Carcinoma . BioMed Research International. 2018.
- Perera IR, Attygalla M, Jayasuriya N, Dias DK, Perera ML. Oral hygiene and periodontal disease in male patients with oral cancer. Br
J Oral Maxillofac Surg. 2018; 56: 901-903.
- Siriwardena BSMS, Tilakaratne A, Amaratunga EAPD, Tilakaratne
WM. Demographic, aetiological and survival differences of oral
squamous cell carcinoma in the young and the old in Sri Lanka.
Oral Oncol. 2006; 42: 831-836.
- Wong YK, Tsai WC, Lin JC, Poon CK, Chao SY, Hsiao YL, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral
Oncol. 2006; 42: 893-906.
- Mahmood N, Hanif M, Ahmed A, Jamal Q, Saqib, Khan A. Impact of
age at diagnosis on clinicopathological outcomes of oral squamous
cell carcinoma patients. Pak J Med Sci. 2018; 34: 595-599.
- Warnakulasuriya S, Mak V, Möller H. Oral cancer survival in young
people in South East England. Oral Oncol. 2007; 43: 982-986.
- Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young
patients: a matched analysis. Oral Oncol. 2007; 43: 894-897.
- Amarasinghe AAHK, Usgodaarachchi US, Johnson NW, Warnakula-suriya S. High Preva-lence of Lifestyle Factors Attributable for Oral
Cancer, and of Oral Potentially Malignant Disorders in Rural Sri
Lanka. Asian Pac J Cancer Prev. 2018; 19: 2485-2492.
- Smits RW, Koljenović S, Hardillo JA, Ten Hove I, Meeuwis CA, et al.
Re-section margins in oral cancer surgery: Room for improvement.
Head Neck. 2016; 38: E2197-203.
- de Araújo RF Jr, Barboza CA, Clebis NK, de Moura SA, Lopes Costa
Ade L. Prognostic significance of the anatomical location and TNM
clinical classification in oral squamous cell carcinoma. Med Oral
Patol Oral Cir Bucal. 2008; 13: E344-E347.
- Lin CS, Jen YM, Cheng MF, Lin YS, Su WF, et al. Squamous cell carcinoma of the buccal mucosa: an aggressive cancer requiring multi-modality treatment. Head Neck. 2006; 28: 150-157.
- Shaw RJ, McGlashan G, Woolgar JA, Lowe D, Brown JS, et al. Prognostic importance of site in squamous cell carcinoma of the buccal
mucosa. Br J Oral Maxillofac Surg. 2009; 47: 356-359.
- Chhetri DK, Rawnsley JD, Calcaterra TC. Carcinoma of the buccal
mucosa. Otolaryngol Head Neck Surg. 2000; 123: 566-571.
- Mitchell DA, Kanatas A, Murphy C, Chengot P, Smith AB, et al. Margins and sur-vival in oral cancer. Br J Oral Maxillofac Surg. 2018; 56:
820-829.
- McMahon J, O’Brien CJ, Pathak I, Hamill R, McNeil E, et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral
Maxillofac Surg. 2003; 41: 224-231.
- Barry CP, Ahmed F, Rogers SN, Lowe D, Bekiroglu F, et al. Influence
of sur-gical margins on local recurrence in T1/T2 oral squamous
cell carcinoma. Head Neck. 2015; 37: 1176-1180.
- Weijers M, Snow GB, Bezemer DP, van dr Wal JE, van der Waal
I. The status of the deep surgical margins in tongue and floor of
mouth squamous cell carcinoma and risk of local recurrence; an
analysis of 68 patients. Int J Oral Maxillofac Surg. 2004; 33: 146-149.
- Wong LS, McMahon J, Devine J, McLellan D, Thompson E, et al.
Influence of close resection margins on local recurrence and disease-specific survival in oral and oro-pharyngeal carcinoma. Br J
Oral Maxillofac Surg. 2012; 50: 102-108.
- Chiou WY, Lin HY, Hsu FC, Lee MS, Ho HC, et al. Buccal mucosa
carcinoma: surgical margin less than 3 mm, not 5 mm, predicts
locoregional recurrence. Radiat On-col. 2010; 5: 79.
- Dik EA, Willems SM, Ipenburg NA, Adriaansens SO, Rosenberg AJ,
et al. Resec-tion of early oral squamous cell carcinoma with positive or close margins: relevance of ad-juvant treatment in relation
to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol.
2014; 50: 611-615.
- Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, et al. A Proposal to Redefine Close Surgical Margins in Squamous Cell Carcinoma of the Oral Tongue. JA-MA Otolaryngol Head Neck Surg. 2017;
143: 555-560.
- Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sándor GK. What is
the adequate margin of surgical resection in oral cancer? Oral Surg
Oral Med Oral Pathol Oral Radi-olEndod. 2009; 107: 625-629.
- Anderson CR, Sisson K, Moncrieff M. A meta-analysis of margin
size and local recur-rence in oral squamous cell carcinoma. Oral
Oncol. 2015; 51: 464-469.