Introduction
Johne’s disease in ruminants is caused by Mycobacterium
avium subsp. Paratuberculosis (MAP), a persistent rubor with
significant economic effects and global dissemination [1]. The
apparent correlation between Mycobacterium avium subspecies
paratuberculosis and Crohn’s disease in individuals is still being
studied extensively, with conflicting results [2-4]. In 1895, German
researchers Johne and Frothingham acknowledged MAP for the
first time [5]. It commonly infects ruminants (cattle, sheep, goats,
deer, and so on) (Figure 1), however, it has also been reported
in non-ruminants, notably wildlife [6]. Annual cattle sector losses
in the United States have been estimated to be between $250
million [7] and $1.5 billion [8]. According to a new assessment of available data employing a Bayesian technique [9], calibrated
for susceptivity and explicitness, the underlying frequency of
MAP in dairy cattle in the United States was 91.1%, not 70.4%
claimed in 2007 [10]. The incidence of MAP in beef cattle herds
is 7.9% [11]. Even though JD was initially discovered in the United
States during the early 1900s, the emphasis on investigation and
disease prevention alone has expanded in the last 20 years. To
combat Johne’s disease on a farm as well as to recognize herds
having minimal infection susceptibility, a discretionary Bovine
JD Management Program is in operation. The examination of
ambient stool specimens via culturing through elevated sites is
among the most cost-effective as well as highly reliable diagnostic
techniques for JD [9]. Ironically, wildlife repositories may disrupt
initiatives to reduce Johne’s disease in livestock unless their
significance in wildlife is completely defined [13]. JD transmission
is reduced when improved diagnoses are combined with good
management strategies [12].
Taxonomy and properties
The Mycobacterium avium complex, which belongs to the
genus Mycobacterium and the family Mycobacteriaceae, contains
MAP. Mycobacterium avium and Mycobacterium intracellulare
are two distinct species in the Mycobacterium avium complex.
Mycobacterium avium subsp. avium, Mycobacterium avium
subsp. hominissuis (MAH), MAP, and Mycobacterium avium
subsp. silvaticum are the four subspecies of M. avium, according
to a thorough sequence-based evaluation of the internal
transcribed spacer of 16S-23S ribosomal RNA [14,15]. MAP is a
gram-positive, acid-fast, rod-shaped intracellular bacteria with
a diameter of 0.5 to 1.5 m. The bacterial cell wall is dense and
waxy arabinogalactan holds the mycolate and peptidoglycan
layers intact. Bacteria is a slow-growing that takes over 20 hrs. to
multiply [16]. Efforts to build up MAP in the research lab medium
were initially unsuccessful [17], and it was hypothesized that
MAP’s failure to cultivate in-vitro was due to a scarcity of a crucial
development factor. Further analysis revealed that MAP could
flourish on a medium enriched with extracts from many other
mycobacteria [18,19], leading scientists to assume that MAP
cannot generate a vital growth factor that some other species
can synthesize. Mycobactin is a siderophore that binds iron and
is produced from Mycobacterium phlei, which has been identified
as the growth factor required for MAP cultivation in-vitro [20,21].
Mycobactin dependence has been regarded as taxonomic for MAP
since that period. A mission in the mbtA gene in the mycobactin-production operon has recently revealed a molecular knowledge
of mycobactin reliance, as explained further below with the
genome sequence [22,23].
Pathogenesis
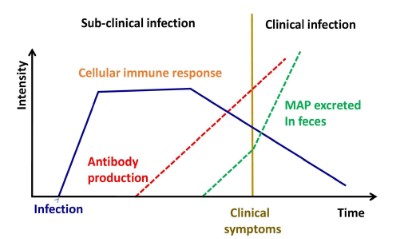
Johne’s Disease (JD) is characterized by persistent diarrhea
and a malabsorption condition, which results in malnutrition
and muscle atrophy (Figure 2A). The faeco-oral pathway is the
most common way for neonates and young animals to become
infected. Milk feeding from infected dam is another source of
infection to neonates [24]. Calves up to the age of six months have
a greater incidence of infection, but afterward, the risk reduces
[25]. According to animal research, M-cells and enterocytes
both promote MAP adjunct to and transit through the gut
mucosa upon consumption [26]. Tissue culture observations demonstrate that MAP influences the establishment of tight
junctions in the intestinal mucosa, offering a mechanism for
enhanced permeability [27] (Figure 2). Antigens 85 [28], 35 kDa
[29], MAP oxidoreductase [30], MAP fibronectin-binding protein
[31,32], and histone HupB [33] are all crucial in MAP epithelial cell
adhesion and/or penetration, and there is a lot of host-pathogen
interaction going on. Prior literature has shown that phagosome
acidification stimulates interleukin (IL)-1 production, macrophage
recruitment, and trans-epithelial migration in MAP-infected
epithelial cells utilizing the cow mammary epithelial cell line MAC-T
[34] and bovine Blood-Monocyte-Derived Macrophages (BMDM)
[35]. Bacilli (genus Bacillus) are subsequently phagocytosed in
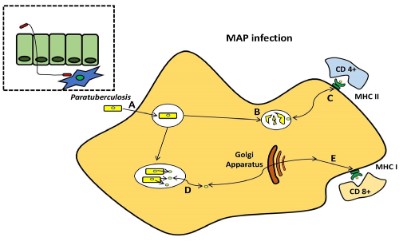
the sub- and intraepithelial spaces by these macrophages [36-38]. For pathogenesis, MAP’s capacity to persist and proliferate
once inside phagocytic cells is fundamental [39,40]. Furthermore,
researchers observed that the lipid content of MAP changes in
macrophages that acquire a pro-inflammatory phenotype utilizing
a culture passage model (Figure 3) [41].
The pathognomonic granulomatous enteritis of Johne’s illness
[38], which is characterized by a wide and ridged intestinal wall
as well as inflammatory lymph nodes, is the result of the ensuing
host cellular immunological response. Toll-like receptors help
tissue macrophages and dendritic cells recognize molecular
patterns linked with pathogens in the innate phase, as well as the
abstraction of cytokine-mediated cellular connections and antigen
processing [42,43]. In the acquired immunity phase, Th1 T-helper
cell responses and concurrent stimulation of macrophages by
Interferon-Gamma (INF) produced by Th1 T cells are used to
reduce MAP infections [44,45]. The inferential function of nitric
oxide synthase, has already been shown in cattle, is implicated
in the killing process of these activated phagocytic cells [46]. In
this condition, BMDM recovered from sub-clinically contaminated
animals exhibits exceptionally high levels of nitric oxide generation
(Figure 4) [47]. MAP, on the other hand, affects the activity
of bovine macrophages, as demonstrated by distinct profiles
of mRNA expression [48], apoptosis suppression and antigen
distribution [49], and diagnostic cytokine expression patterns
[50]. In infected bovine T helper cells, MAP mostly generates a
Th2 response, with increased production of IL-4, IL-5, IL-10, and
tissues remodeling inhibitors [51,52]. This humoral response
was confirmed in a newborn calf model [53]. In addition, in both
ruminants and animals, regulatory T and Th17 cells have been
involved in the immune pathogenesis of JD [49,54].
MAP pathogenesis has been studied using a variety of models.
MAP, on the other hand, produces immunological responses in
ruminant hosts not found in traditional in vitro models. MAP
bacilli grow during 4–8 days in infected BMDM [44,55], although
bacterial burdens are reduced over time after infection of the
murine J774 macrophage cell line [44,55-57]. When researching,
the interactions between MAP and phagocytic cells, it is preferable
to use primary phagocytic cells. To follow the progression of
MAP infection from initial to final stages, Ileal loops have been
employed to establish a prospective systems biology approach
[58]. The host transcriptome profile following infection with M.
avium subsp. avium and MAP were recently compared using
this paradigm. Intestinal mucosal weakening, activation of a Th2
reaction, and phagocytosis suppression were all related to MAP
transmission, which was not found with M. avium subsp. avium
infection (Figure 5) [59].
Diagnosis and control
Before any clinical indications, infected animals shed MAP in
their feces, making them a prominent cause of infection forthe
herd's other animals. To avoid the spread of JD, it is critical to diagnose the infection as soon as possible. Based on the detection of
MAP both directly and indirectly, many diagnostic tests have been
created [60]. Direct identification of MAP in clinical specimens can
be thriving using (i) microscopy, (ii) culture-based MAP isolation,
and (iii) PCR-based MAP DNA identification. Clinical samples have
been analyzed using acid-fast staining or Ziehl–Neelsen. Acid-fast
staining is the easiest, quickest, and also a most economical mode
of diagnosis, but its accuracy and precision are inadequate since
it is challenging to discern between MAP and some other acid-fast
bacilli [61]. Although Ziehl–Neelsen staining can also be used to
screen for MAP; it must be verified by additional procedures such
as PCR and/or immunoassays. The "gold standard" for JD diagnosis is MAP isolation through culture. The fact that MAP requires
mycobactin J to grow in a specific laboratory medium can be utilized to distinguish it from many other acid-fast bacteria. A novel
growth media that increases MAP restoration and sensitivity by
1,000-fold was recently divulged [62]. Because MAP develops
slowly (On solid medium, colony development takes 6–8 weeks.),
culture-based diagnosis takes a long period. As a consequence,
a highly fast and precise PCR-based test was employed for MAP
identification in environmental and clinical specimens [63-65].
IS900 is a 1.4 kb multi-copy insertion element that is sequence
specific to MAP. The primers used in this PCR are for IS900 [60,
66]. Other mycobacteria with IS900-like insertion sequences, on
the other hand, have been demonstrated to influence the specificity of this test, resulting in false-positive findings [64,67]. To
prevent false-positive results, a multiplex PCR centered on the
IS900, IS901, IS1245, and dnaJ genes was constructed, although
the precision of this assay is restricted owing to reagent interference and primer-dimer generation [60, 68]. Furthermore, PCR
tests based on stool specimens hold only 70% sensitivity and 85%
specificity [69]. There has been some advancement in identifying and utilizing more precise targets for PCR testing [70-72], and
this comparative genomic technique has addressed an apprehension gap in MAP identification. Several of these objectives have
made their way into commercial diagnostic tools.
The immunological response of the host to infection is the basis for diagnostic MAP tests based on indirect detection. A Johnin
pure protein derivative was used to produce the delayed-type
hypersensitivity skin test [73]. However, because various environmental mycobacteria might sensitize the animal and provide false-positive findings, this test is not specific. As a result, delayed-type
hypersensitivity skin tests can't tell the difference between vaccinated and animals that have been naturally affected. As previously established, MAP invasion triggers T helper cells, which secrete
IFN-γ. The utilization of cultures supernatants from day-old blood
specimens treated with Johnin and co-stimulated with human IL-2and/or bovine IL-12 can also be used to diagnose JD using an
enzyme-linked immune sorbent test (ELISA) [74]. Unfortunately,
cross-reactivity issues arise because in the INF-test, MAP pure
proteins analogs are often used as antigens. A potential alternative MAP antigen for the research was L5P, a cell wall lipopeptide,
however, the IFN-γ expression was reported to be weaker than
that of Johnin [75]. Antibodies in milk and serum from diseased
animals are detected using commercial ELISA kits such as (I)) Para
Check (CSL/Biocor), (ii) Herd Check M. paratuberculosis ELISA
(IDEXX Laboratories, Inc.), (iii) ID Screen® Paratuberculosis Indirect (ID Screen® Paratuberculosis Indirect (ID Screen (IDvet Genetics) and (iv) SERELISA ParaTB (Synbiotic Corp.). In comparison to
PCR testing, an ELISA seems to have a lower sensitivity of 50% but
a far higher specificity of 99.8% [76,77]. To establish better sensitive immune-based tests for JD diagnosis, additional research is
needed to uncover MAP specialized antigens.
Vaccination (the most economical), screening, and improved
herd control are all alternatives for avoiding JD, depending on a
producer's finances, infrastructure, and operations [78]. However,
while JD vaccines can diminish systemic disease and discharge,
their effectiveness is minimal, and none of them provides fairly
long immunity. In the United States, for instance, Mycopar®
(BoehringerIngelheim Vetmedica, Inc.) has been exclusively licensed vaccination for JD in cattle. Unfortunately, since strain 18
of M. avium subsp. avium was used to make the vaccine [79], but
it lacks an ideal antigenic repertoire. In Australia, Silirum® (Zoetis
Animal Health), a different bacterin, is being investigated and it
has been licensed for restricted usage in cattle. The MAP 316F
strain has been heat-killed in this vaccination. This formulation
may contain a broader spectrum of antigenic, however utilizing
bacteria that have been destroyed by heat, may lower efficacy
while improving safety. Both Neoparasec® (Rhone-Merieux) and
Gudair® (Zoetis Animal Health) contain the live-attenuated MAP
strain 316F and are authorized for usage in goats and sheep. Vaccines that are currently available, on the other hand, are unable
to discriminate between vaccinated and infected animals, impairing JD diagnostic testing [80], and strain 316F was created in the
1920s using random depreciation processes (e.g., passages in ox
bile) that are currently being examined [81]. Eventually, to successfully manage JD, an elevated vaccination is necessary [82].
Human anti-tuberculosis vaccines of the latest era appear to
provide higher protection than subunit vaccines, according to
testing results [83]. Because JD is induced by a bacteria called Mycobacterium, potential subunit or bacterin-based vaccines are
likely to face a similar situation. The JD Integrative Protocol-Animal and Plant Health Inspection Service's endeavors to establish
a consistent vaccination testing program were spurred by this. In
a three-phase investigation, investigators from New Zealand and
the U.S provided 22 masked live-attenuated immunization candidates to be evaluated in mouse, BMDM, and goat models. Despite
the substantial development of animal screening procedures [84],
the bulk of the suppressed transposon variants investigated was
the first generation and had the Tn5367 transposase, that caused
destabilization. Furthermore, unknowns including the ideal immunization path and dose plan could not be determined before
the commencement of the experiment. Despite this, crucial information and chemicals were created [80]. It is yet conceivable to
design a subunit vaccine that can manage infections by inducing
the appropriate humoral immunity [85], specifically against antigens produced by the pro-inflammatory phenotype [86].
However in absence of a vaccine, control of MAP infection in
the human population can be accomplished, either by surgical removal of infected intestines or by medicines [87] using anti-tuberculosis drugs, which had limited success [88,89]. The Prolonged
use of anti-tuberculosis drugs led to the development of drug resistance to all the existing anti-mycobacterial molecules. Because
of the increase in cases of animal and human infections, demand
for natural products as an alternative therapy for this chronic incurable disease has increased. This has encouraged researchers to
find out bio-active (marker) compounds from plants with pharmacological properties against symptoms exhibited by MAP-infected
domestic livestock populations, e.g., chronic progressive inflammation, etc. Earlier studies have suggested that plant extracts
have possible feasibility to decreasing induction of TNF-α that
can modulate TNF-α mediated inflammatory pathways and may
have potential against diseases arising due to chronic inflammation caused by MAP infection (paratuberculosis or Johne’s disease
in animals and Crohn's disease in humans). Plants extracts play
a major role as immuno-modulator and immuno-stimulator and
can increase or decrease the level of various pro-inflammatory
and inflammatory cytokines during chronic inflammation.
The pre-2nd century 'Charaka Samhita' book reported Ayurveda
(Indian traditional medicine) Herbal medicinal plants have been
used to cure tuberculosis including various ailments. Decoctions,
Infusions, Tinctures and macerations of Herbal medicinal plants
parts such as fruits and flowers, stem bark, roots, stems, and
leaves have been used for traditional treatment for many century's TB by native people worldwide. Even though ethnopharmacological and ethnobotanical studies consider wide use in the
treatment of TB, most of them were established still to be therapeutic and safe doses. Most of the research studies have failed to
give scientific proof to therapeutic practices and traditional beliefs. Consequently, this work is an endeavor to archive traditionally medicinal plants used to control TB. Contrasting traditional
therapeutic systems used to have been applied to cure TB, going
from the poorly documented oral Indian medicine to the well-documented Indian, Ayurveda and so on.
Description of Ocimum sanctum plant
Taxonomic classification of Ocimum sanctum plant
Scientific Name: Ocimum sanctum
AyurvedicName: Tulsi
Division: Magnoliophyta
Class: Magnoliopsida
Subclass: Asteridae
Order: Lamiales
Family: Lamiaceae
Genus: Ocimum
Morphology
Tulsi (Ocimum sanctum) is an upright, multi-branched sub-shrub, 300–600 mm (30-60 cm) tall with hairy stems. Leaves color
is purple or green; the petioled, with up to 5 cm (2 inches) long
and ovate blade and also a slightly toothed margin; the plant fragrant is very strong and Phyllotaxy is decussate. The flowers are
purplish and placed in close whorls on elongated racemes [90]. In
India and Nepal, three main types of morphotypes are cultivated
that is Ram tulsi (which is a common type with broad bright slightly sweet green leaves). Purplish green-leaved is less common in
Krishna or Shyamtulsi and Vana tulsiis the common in wild [91].
Soil and climate
Ocimum sanctum (Holy basil) plant can be grown in moderately
shaded conditions with low oil contents. Waterlogged conditions
can cause root rot and growth to be stunted. It well flourishes
under high rainfall and humid conditions. The high temperatures
and long days have been found favorable for plant growth and
oil production. Soil & Manure: Porous, aerated, and well-drained
with added organic manure of soil is required for plant growth.
Clay & Sticky soil is not good for the plant's roots.
Floral characteristics
Ocimum sanctum plant is a short-lived perennial shrub or small annual, up to 3.3 feet (100 cm) in height. The simple toothed and
hairy stems are oppositely entire leaves along with the stem. The
scented leaves are purple or green, depending upon the variety.
The white tubular or small purple flowers have green or purple
sepals and are supported by terminal spikes. The nut-lets fruits
and numerous seeds are produced.
Propagation
Ocimum sanctum crop can be propagating through the seeds
and sown in the nursery beds. 300 g of seeds are required in one
hectare for the sowing. The nursery should be located in partial
shade with sufficient irrigation facilities and soil depth up to 30
cm. well organic manure is applied to the soil and prepared to a
seed beds size is 4.5 x 1.0 x 0.2 m. As seed quantity is mixed with
the sand ratio is 1:4 required for sown in a nursery bed and 60
days advance in the onset of monsoon. The 8-12 days seeds can
germinate and transplant seedlings in about 6 weeks during the
4-5 leaf stage.
Distribution
The Holy basil plant is widely distributed throughout India and
Central University of Punjab and Bathinda researchers have done
research from the study of large-scale phylogeny graphical of this
species using chloroplast whole genomic sequencing then team
revealed that this holy basil plant originates from North-Central
India [92].
Ocimum sanctum is a native herb in India, and also known as
‘Tulsi’ belongs to the family Lamiaceae. The Hindu religious tradition is sacred and is viewed as perhaps the most significant plant
used in Ayurvedic medicine [93]. Tulsi plants grow in abundance
around Hindu temples. Found in so many varieties strong like
green and a red, pleasant aroma. In the previous decade several
scientific shreds of evidence have been reported [94,95,96] at holy
basil has been utilized to treat a variety of many critical diseases
[97] including asthma, arthritis, heart problems, eye disorders,
blood glucose levels, hepato protective, anticancer, anti-fungal, antimicrobial, chronic fever, anti-fertility and bronchitis [98,99]
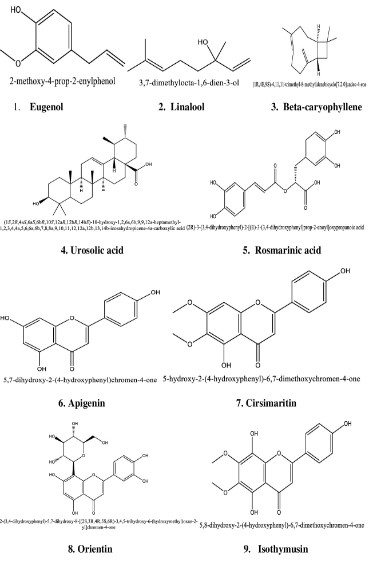
(Table 1) Ocimum sanctum have in so many chemical constituents
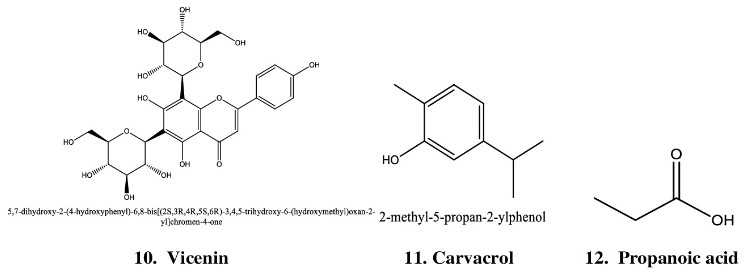
such as carvacrol, eugenol, limatrol, linalool, ursolic acid, caryophyllene, propionic acid, methyl carvicol, Rosmarinic acid, Apigenin, cirsimaritin, Orientin, isothymusin and Vicenin [Figures S1,
S2]. Previous research also showed that the Tulsi leaf juice shows
complete growth inhibition of Anti-viral and Anti-Mycobacterial
activities [100,101].
Table 1: Biological Mechanism between Bioactive constituents with MIC50 Values.
| S.No. |
Bioactive constituents |
MIC50 Value |
Mechanism |
References |
| 1 |
Eugenol |
500 μg/ml |
Antifungal |
Ahmad et al., 2015 [102] |
| 2 |
Linalool |
0.12% |
Antimicrobial |
Federman et al., 2016 [103] |
| 3 |
Ursolic acid |
32 μg/mL
64 μg/mL |
Antimicrobial |
Do Nascimento et al., 2014[104] |
| 4 |
beta-caryophyllene |
32 μg/ml
1024 μg/ml |
Antimicrobial |
Santos et al., 2021 [105] |
| 5 |
Propionic acid |
0.25%
0.125% |
Antimicrobial
Antifungal |
Haque et al., 2009 [106] |
| 6 |
Rosmarinic acid |
1.2 mg/ml
0.3 mg/ml
2.5 mg/ml |
Antimicrobial
Antifungal
Antiviral |
Abedini et al., 2013 [107] |
| 7 |
Apigenin |
>4 mg/ml |
Antimicrobial |
Nayaka et al., 2014 [108] |
| 8 |
Orientin |
500 μg/ml
1000 μg/ml |
Antimicrobial |
Karpiński et al., 2019 [109] |
| 9 |
Isothymusin |
200 μg/mL |
Antimicrobial |
https://www.chemfaces.com/natural/Isothymusin-CFN97562.html [110] |
| 10 |
Vicenin-2 |
>188μg/mL |
Antimicrobial
Antifungal |
Mohotti et al., 2020 [111] |
Table 2: Biological mechanism between bioactive constituents with MIC50 values
| S.No. |
Bioactive constituents |
MIC50 Value |
Mechanism |
References |
| 1 |
Chlorogenic Acid |
20 to 80 μg/mL |
Antibacterial |
Lou et al., 2011 [118] |
| 2 |
Stigmasterol glucoside |
0.67 mg/ml |
Antibacterial |
Swain and Padhy et al., 2015 [119] |
| 3 |
3,4-dihydroxy cinnamic acid methyl ester |
50- 200 μg/mL |
Antibacterial |
Hua Du1 et al., 2009 [120] |
| 4 |
Solasodine |
62.5 μg/mL |
Antibacterial |
Sinani and Eltayeb et al., 2017 [121] |
| 5 |
Solanine |
240μg/mL
120μg/mL
90μg/mL |
Antifungal
Antiviral
Antibacterial |
Kumar P et al., 2009 [122]/ |
| 6 |
Cycloartanol |
8 µg/mL |
Antibacterial |
Woldemichael et al., 2004 [123] |
| 7 |
Stigmesterol |
3.13μg/mL
6.25 μg/mL |
Antibacterial |
Mailafiya et al., 2018 [124] |
| 8 |
Beta-Sitostero |
6.25 µg/ml
12.5 µg/ml |
Antibacterial |
NWEZE et al., 2019 [125] |
| 9 |
Apigenin |
> 4 mg/mL |
Antibacterial |
Nayaka et al., 2014 [126] |
| 10 |
Esculestin |
192 mg/mL
<0.015625 μg/mL |
Antibacterial
Antifungal |
Pushpanathan M et al., 2013 [127] |
| 11 |
Esculin |
2500 mg/L
625 mg/L |
Antibacterial
Antifungal |
Mokdad-Bzeouich et al., 2014 [128] |
| 12 |
Scopoletin |
50 μg/mL (without sorbitol)
>200 μg/mL (with sorbitol) |
Antifungal |
Lemos et al., 2020 [129] |
Description of Solanum xanthocarpum Plant
Taxonomic classification of Solanum xanthocarpumplant
Scientific Name: Solanum xanthocarpum
AyurvedicName: Kantakari
Division: Magnoliophyta
Class: Magnoliopsida
Subclass: Asteridae
Order: Solanales
Family: Solanaceae
Genus:Solanum
Morphology
Solanum xanthocarpum plant is a very thorny diffused bright
green perennial herb, at the base is woody. Branches are several
and spreading on the ground, the new branches are covered
with dense stellate tomentum, yellow, straight, glabrous, prickles
compressed, shining often exceeding and 13 mm long. Leaves
are 50-100 x 25-57 mm, bearing stellate hairs on both sides of
beneath, ovate or elliptic, Petioles are 13-25 mm long. Sometimes
becoming nearly glabrous with age.
Soil and climate
Solanum xanthocarpum is a hardy plant mainly grown in tropical
and sub-tropical regions. It does adequately over light humus-rich, silty sand to rich loamy soils having pH of 7.0-8.0. Kantakari
is a warm-season crop and also a crop grown over saline lands.
The most favorable temperature range is 21-27oC for its growth
and reproduction. Generally, abundant sunshine is required and
dry weather with a long period of warm. In northern India, from
December to January in this season the crop is adversely affected
due to frost as it causes injury to vegetative parts and in the spring
season plant will be recovered.
Floral characteristics
Kantkari flowers are axillary bud, cymes and bluish-violet.
The curvedhairy stellate with short pedicels, linear-lanceolate,
globose, prickly outside and lobes are 1.1 cm long. Purple Cololla,
lobes deltoid, 20 mm long, acute, hairy outside. 1.5 mm long of
Filament, 8 mm long of anthers, glabrous, oblong-lanceolate and
tiny pores are opening. Style glabrous and ovary is ovoid. The
berry-shaped fruits, 13-20 mm in diameter, are white or yellow
with green veins and the calyx is enlarged. Seeds are 2.5 mm in
diameter, sub-reni form, glabrous, smooth and yellowish-brown.
Distribution
Kantakari plant is widely distributed throughout India. The
dry situation in the Himalayas as weed ascended to 1500 meters.
Abundant by roadsides and wastelands, mainly in Uttar Pradesh,
Rajasthan, Madhya Pradesh, Gujarat and Haryana.
Propagation
The crop is elevated by seed and Yellowish-brown color in
seeds, small size i.e. 0.25 cm in diameter and glabrous. There is
no dormancy period for seeds and can be sown after some days of harvesting. The germination percentage is around 60-70% and
it will take 10-15 days to germinate.
Solanum xanthocarpum is a native herb of India, and also known
as kantkari belongs to the family Solanaceae. It is a thorny, bright
green, perennial plant with woody roots that grow to a height of
2 to 3 meters and is found all over India, primarily in arid regions
as a weed on highway shoulders and waste lands. The 1.3 cm in
diameter, yellow or white berry with green veins, and expanded
calyx-shaped fruits are produced [112]. In the previous decade
much scientific evidence has been reported [113] at kantkari
has been utilized to treat a variety of many critical diseases
including cough, fever, heart diseases, antipyretic, hypotensive,
antiasthmatic, antitumor, anti-anaphylactic, aphrodisiac
activities, wound healing, anti-inflammatory, urinary bladder,
laxative [114], blood glucose levels, hepatoprotective, anticancer,
antifungal, antimicrobial, chronic fever, antifertility and bronchitis
[115] (Table 2) Solanum xanthocarpum have in so many chemical
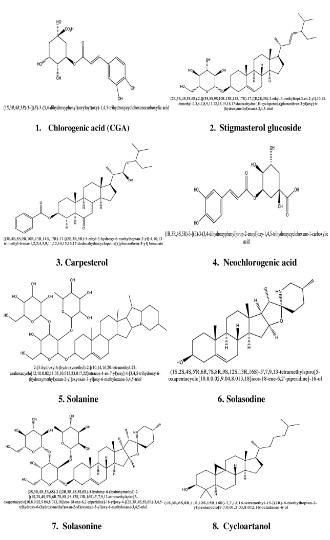
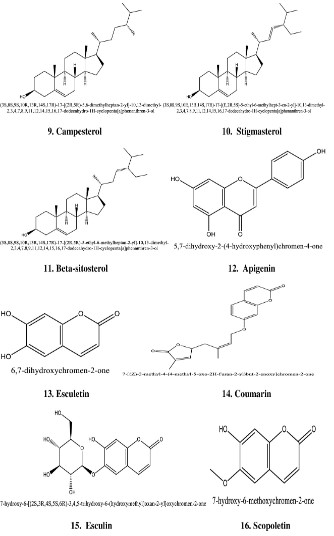
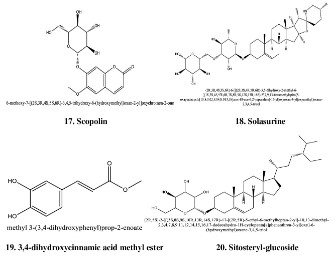
constituents such as chlorogenicacids, stigmasteryl glucoside,
glucoalkaloidsolanocarpine, isochlorogenic, carpesterol, methyl
ester of 3,4-dihydroxycinnamic acid,neochlorogenic cholesterol,
3,4-dihydroxycinnamic acid (caffeic acid), solanine-S, solasodine,
Quercetin 3-O-D-Glucopyranosyl-(1,6)-D-Glucopyranoside,
solasonine, Sitosterol-beta-D-Galactoside, solasurine,
solamargine, cycloartanol, sitosteryl-glucoside, campesterol,
stigmasterol (fruit); sitosterol, flavonal glycoside, apigenin
(flower); amino acids and solanocarpine (seeds); esculetin,
coumarins, esculin, scopolin and scopoletin (leaves, fruits and
roots); norcarpesterol, tomatidenolandcarpesterol (plant) [116]
(Figures S3, S4, S5). Previous researches also showed that the
kantkari fruit juice show complete growth inhibition of Anti-viral
(HIV), anticancer and Anti-Mycobacterial activities [117].
Conclusion
Natural chemicals can be utilized to enhance the efficacy of anti-tuberculosis treatments and perhaps fill in the gaps where regular prescription therapies have lost their effectiveness. Prevention and treatment strategies, combined with natural substances,
may be a feasible alternative for reducing drug resistance. As discussed, natural substances possess a multitude of antimycobacterial characteristics and focus on several therapeutic targets. For
instance, natural compounds can augment the sensitivity of mycobacterium to antibiotic treatment. Natural items should be researched further for the treatment of active TB. It is worth noting
that many of the studies included in this review were carried out
using techniques such as molecular assays, mouse models, animal
cells, and bacterial culture. Natural products must be of excellent
quality, authentic, well formulated, regularly derived from their
sources, and not contaminated with other products. Novel natural
chemicals are being researched in the hope that they will be effective in treating tuberculosis infections.
We emphasize on identifying plants based on ethnomedical
complaints and testing their extracts/phytomolecules against Mycobacterium paratuberculosis strain. In conclusion, we tried to
give brief idea about those natural compounds found suitable to
paraphrase research activity against paratuberculosis. In a result
we can say that two plants extract can achieve good combination effect, although any antagonistic effect was not determined
yet. Therefore, targeting these two agents will help in future to
shorten the current therapeutic regimens for para TB and also for
treating other tuberculosis diseases also.
Conflict of interest: There is no conflict of interest to declare.
References
- Sweeney RW. Transmission of paratuberculosis. In American Association of Bovine Practitioners Conference Proceedings. 1994;
72-74.
- Kaufmann SH, Follows GA, Munik ME. Immunity to intracellular
bacteria. Memórias do Instituto Oswaldo Cruz. 1992; 87: 91-4.
- Davis WC, Madsen-Bouterse SA. Crohn’s disease and Mycobacterium avium subsp. paratuberculosis: the need for a study is long
overdue. Veterinary immunology and immunopathology. 2012;
145: 1-6.
- Momotani E, Romona NM, Yoshihara K, Momotani Y, Hori M, Ozaki
H, Eda S, Ikegami M. Molecular pathogenesis of bovine paratuberculosis and human inflammatory bowel diseases. Veterinary immunology and immunopathology. 2012; 148: 55-68.
- Johne H, Frothingham L. A particular case of tuberculosis in a cow.
Dtsch Z Tiermed Pathol. 1895; 21: 438-454.
- Motiwala AS, Amonsin A, Strother M, Manning EJ, Kapur V, et al.
Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolates recovered from wild animal species. Journal of
clinical microbiology. 2004; 42: 1703-1712.
- Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated
with Johne’s disease on US dairy operations. Preventive veterinary
medicine. 1999; 40: 179-192.
- Stabel JR. Johne’s disease: a hidden threat. Journal of dairy science. 1998; 81: 283-288.
- Lombard JE, Gardner IA, Jafarzadeh SR, Fossler CP, Harris B, et al.
Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Preventive
Veterinary Medicine. 2013; 108: 234-238.
- Dairy US. Part I: Reference of dairy cattle health and management
practices in the United States, 2007. Fort Collins, CO: USDA-APHIS.
2007.
- Lombard JE. Epidemiology and economics of paratuberculosis.
Veterinary Clinics: Food Animal Practice. 2011; 27: 525-535.
- Espejo LA, Godden S, Hartmann WL, Wells SJ. Reduction in incidence of Johne’s disease associated with implementation of a disease control program in Minnesota demonstration herds. Journal
of dairy science. 2012; 95: 4141-4152.
- Miller RS, Farnsworth ML, Malmberg JL. Diseases at the livestock–wildlife interface: status, challenges, and opportunities in the United States. Preventive veterinary medicine. 2013; 110: 119-132.
- Frothingham R, Wilson KH. Sequence-based differentiation of
strains in the Mycobacterium avium complex. Journal of Bacteriology. 1993; 175: 2818-2825.
- Mijs W, de Haas P, Rossau R, Van Der Laan T, Rigouts L, et al. Molecular evidence to support a proposal to reserve the designation
Mycobacterium avium subsp. avium for bird-type isolates and’M.
avium subsp. hominissuis’ for the human/porcine type of M. avium. International journal of systematic and evolutionary microbiology. 2002; 52: 1505-1518.
- Lambrecht RS, Carriere JF, Collins MT. A model for analyzing growth
kinetics of a slowly growing Mycobacterium sp. Applied and environmental microbiology. 1988; 54: 910-916.
- Zhu W, Arceneaux JE, Beggs ML, Byers BR, Eisenach KD, Lundrigan
MD. Exochelin genes in Mycobacterium smegmatis: identification
of an ABC transporter and two non-ribosomal peptide synthetase
genes. Molecular microbiology. 1998; 29: 629-639.
- Rathnaiah G, Zinniel DK, Bannantine JP, Stabel JR, Gröhn YT, Collins
MT, Barletta RG. Pathogenesis, molecular genetics, and genomics
of Mycobacterium avium subsp. paratuberculosis, the etiologic
agent of Johne’s disease. Frontiers in veterinary science. 2017; 4:
187.
- Twort FW, Ingram GL. Further experiments on the biology of Johne’s bacillus. Zentr Bakteriol Parasitenk Abt 1 Org. 1914;73:277-83.
- Snow GA. Isolation and structure of mycobactin T, a growth factor
from Mycobacterium tuberculosis. Biochemical Journal. 1965; 97:
166-175.
- Francis J, Macturk HM, Madinaveitia J, Snow GA. Mycobactin, a
growth factor for Mycobacterium johnei. 1. Isolation from Mycobacterium phlei. Biochemical Journal. 1953; 55: 596.
- Snow GA. Isolation and structure of mycobactin T, a growth facto
Windsor PA, Whittington RJ. Evidence for age susceptibility of cattle to Johne’s disease. The Veterinary Journal. 2010; 184: 37-44.
- Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, et al. The complete genome sequence of Mycobacterium avium subspecies
paratuberculosis. Proceedings of the National Academy of Sciences. 2005; 102: 12344-12349.
- Bannantine JP, Bermudez LE. No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis.
Infection and immunity. 2013; 81: 3960-3965.
- de Kruijf M, Coffey A, O’Mahony J. The investigation of the truncated mbtA gene within the mycobactin cluster of Mycobacterium
avium subspecies paratuberculosis as a novel diagnostic marker
for real-time PCR. Journal of Microbiological Methods. 2017; 136:
40-48.
- Streeter RN, Hoffsis GF, Bech-Nielsen S, Shulaw WP, Rings DM.
Isolation of Mycobacterium paratuberculosis from colostrum and
milk of subclinically infected cows. American journal of veterinary
research. 1995; 56: 1322-1324.
- Windsor PA, Whittington RJ. Evidence for age susceptibility of cattle to Johne’s disease. The Veterinary Journal. 2010; 184: 37-44.
- Bermudez LE, Petrofsky M, Sommer S, Barletta RG. Peyer’s patch-deficient mice demonstrate that Mycobacterium avium subsp.
paratuberculosis translocates across the mucosal barrier via both
M cells and enterocytes but has inefficient dissemination. Infection and immunity. 2010; 78: 3570-3577.
- Bannantine JP, Bermudez LE. No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis.
Infection and immunity. 2013; 81: 3960-3965.
- Kuo CJ, Bell H, Hsieh CL, Ptak CP, Chang YF. Novel mycobacteria antigen 85 complex binding motif on fibronectin. Journal of Biological Chemistry. 2012; 287: 1892-1902.
- Bannantine JP, Huntley JF, Miltner E, Stabel JR, Bermudez LE. The
Mycobacterium avium subsp. paratuberculosis 35 kDa protein
plays a role in invasion of bovine epithelial cells. Microbiology.
2003; 149: 2061-2069.
- Alonso-Hearn M, Patel D, Danelishvili L, Meunier-Goddik L, Bermudez LE. The Mycobacterium avium subsp. paratuberculosis
MAP3464 gene encodes an oxidoreductase involved in invasion of
bovine epithelial cells through the activation of host cell Cdc42.
Infection and immunity. 2008; 76: 170-178.
- Secott TE, Lin TL, Wu CC. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium
subsp. paratuberculosis. Infection and immunity. 2001; 69: 2075-2082.
- Secott TE, Lin TL, Wu CC. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by
Mycobacterium avium subsp. paratuberculosis. Infection and immunity. 2002; 70: 2670-2675.
- Rathnaiah G, Zinniel DK, Bannantine JP, Stabel JR, Gröhn YT, et al.
Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne’s
disease. Frontiers in veterinary science. 2017; 4: 187.
- Patel D, Danelishvili L, Yamazaki Y, Alonso M, Paustian ML, et al.
The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infection and Immunity. 2006; 74: 2849-2855.
- Koets AP, Eda S, Sreevatsan S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: where
time and place matter. Veterinary research. 2015; 46: 1-7.
- Momotani E, Whipple DL, Thiermann AB, Cheville NF. Role of M
cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Veterinary
pathology. 1988; 25: 131-137.
- Fujimura Y, Owen RL. M cells as portals of infection: clinical and
pathophysiological aspects. Infectious agents and disease. 1996;
5: 144-156.
- Lugton IW. Mucosa-associated lymphoid tissues as sites for uptake,
carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunology and cell biology. 1999; 77: 364-372.
- Kaufmann SH. Immunity to intracellular bacteria. Annual review of
immunology. 1993; 11: 129-163.
- Zhao BE, Collins MT, Czuprynski CJ. Effects of gamma interferon
and nitric oxide on the interaction of Mycobacterium avium subsp.
paratuberculosis with bovine monocytes. Infection and Immunity.
1997; 65: 1761-1766.
- Everman JL, Eckstein TM, Roussey J, Coussens P, Bannantine JP, et
al. Characterization of the inflammatory phenotype of Mycobacterium avium subspecies paratuberculosis using a novel cell culture
passage model. Microbiology. 2015; 161: 1420-1434.
- Chamy ELL, Leclerc V, Caldelari I, Reichhart JM. Sensing of “Danger
Signals” and Pathogen Associated Molecular Patterns Defines Binary Signaling Pathways “Upstream” of Toll. Nature Immunology.
2008; 9: 1165-1170.
- Coussens PM, Verman N, Coussens MA, Elftman MD, McNulty AM.Cytokine gene expression in peripheral blood mononuclear cells
and tissues of cattle infected with Mycobacterium avium subsp.
paratuberculosis: evidence for an inherent proinflammatory gene
expression pattern. Infection and immunity. 2004; 72: 1409-1422.
- Zhao B, Czuprynski CJ, Collins MT. Intracellular fate of Mycobacterium avium subspecies paratuberculosis in monocytes from normal and infected, interferon-responsive cows as determined by
a radiometric method. Canadian journal of veterinary research.
1999; 63: 56.
- Stabel JR. Transitions in immune responses to Mycobacterium
paratuberculosis. Veterinary microbiology. 2000; 77: 465-473.
- Li RW, Li C, Gasbarre LC. The vitamin D receptor and inducible nitric oxide synthase associated pathways in acquired resistance to
Cooperia oncophora infection in cattle. Veterinary research. 2011;
42: 1-0.
- Khalifeh MS, Al-Majali AM, Stabel JR. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium
subsp. paratuberculosis. Veterinary immunology and immunopathology. 2009; 131: 97-104.
- Tooker BC, Burton JL, Coussens PM. Survival tactics of M. paratuberculosis in bovine macrophage cells. Veterinary immunology
and immunopathology. 2002; 87: 429-437.
- Coussens PM, Sipkovsky S, Murphy B, Roussey J, Colvin CJ. Regulatory T cells in cattle and their potential role in bovine paratuberculosis. Comparative immunology, microbiology and infectious
diseases. 2012; 35: 233-239.
- Weiss DJ, Evanson OA, McClenahan DJ, Abrahamsen MS, Walcheck
BK. Regulation of expression of major histocompatibility antigens
by bovine macrophages infected with Mycobacterium avium sub-sp. paratuberculosis or Mycobacterium avium subsp. avium. Infection and immunity. 2001; 69: 1002-1008.
- Coussens PM, Pudrith CB, Skovgaard K, Ren X, Suchyta SP, et al.
Johne’s disease in cattle is associated with enhanced expression
of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral
blood mononuclear cells. Veterinary immunology and immunopathology. 2005; 105: 221-234.
- Weiss DJ, Evanson OA, Souza CD. Expression of interleukin-10 and
suppressor of cytokine signaling-3 associated with susceptibility
of cattle to infection with Mycobacterium avium subsp paratuberculosis. American journal of veterinary research. 2005; 66: 1114-1120.
- Stabel JR, Waters WR, Bannantine JP, Lyashchenko K. Mediation
of host immune responses after immunization of neonatal calves
with a heat-killed Mycobacterium avium subsp. paratuberculosis
vaccine. Clinical and Vaccine Immunology. 2011; 18: 2079-2089.
- Robinson MW, O’brien R, Mackintosh CG, Clark RG, Griffin JF. Immunoregulatory cytokines are associated with protection from immunopathology following Mycobacterium avium subspecies paratuberculosis infection in red deer. Infection and immunity. 2011;
79: 2089-2097.
- Woo SR, Heintz JA, Albrecht R, Barletta RG, Czuprynski CJ. Life and
death in bovine monocytes: the fate of Mycobacterium avium sub-sp. paratuberculosis. Microbial pathogenesis. 2007; 43: 106-113.
- Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, et al.
Characterization of the intracellular survival of Mycobacterium
avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cellular
microbiology. 2001; 3: 551-566.
- Bannantine JP, Stabel JR. Killing of Mycobacterium avium subspecies paratuberculosis within macrophages. BMC microbiology.
2002; 2: 1-7.
- Khare S, Lawhon SD, Drake KL, Nunes JE, Figueiredo JF, et al. Systems biology analysis of gene expression during in vivo Mycobacterium avium paratuberculosis enteric colonization reveals role for
immune tolerance.
- Khare S, Drake KL, Lawhon SD, Nunes JE, Figueiredo JF, et al. Systems analysis of early host gene expression provides clues for transient Mycobacterium avium ssp avium vs. persistent Mycobacte-
rium avium ssp paratuberculosis intestinal infections. PloS One.
2016; 11: e0161946.
- Chaubey KK, Gupta RD, Gupta S, Singh SV, Bhatia AK, et al. Trends
and advances in the diagnosis and control of paratuberculosis in
domestic livestock. Veterinary Quarterly. 2016; 36: 203-227.
- Manning EJ, Collins MT. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Revue scientifique
et technique (International Office of Epizootics). 2001; 20: 133-150.
- Bull TJ, Munshi T, Mikkelsen H, Hartmann SB, Sørensen MR, et al.
Improved culture medium (TiKa) for Mycobacterium avium sub-species paratuberculosis (MAP) matches qPCR sensitivity and reveals significant proportions of non-viable MAP in lymphoid tissue
of vaccinated MAP challenged animals. Frontiers in Microbiology.
2017; 7: 2112.
- Vary PH, Andersen PR, Green E, Hermon-Taylor J, McFadden JJ.
Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne’s disease.
Journal of clinical microbiology. 1990; 28: 933-937.
- Cousins DV, Whittington R, Marsh I, Masters A, Evans RJ, et al. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS 900-like
sequences detectable by IS 900 polymerase chain reaction: implications for diagnosis. Molecular and cellular probes. 1999; 13:
431-442.
- Ellingson JL, Stabel JR, Bishai WR, Frothingham R, Miller JM. Evaluation of the accuracy and reproducibility of a practical PCR panel
assay for rapid detection and differentiation ofMycobacterium avium subspecies. Molecular and cellular probes. 2000; 14: 153-161.
- Harris NB, Barletta RG. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clinical microbiology reviews. 2001;
14: 489-512.
- Englund S, Bölske G, Johansson KE. An IS 900-like sequence found
in a Mycobacterium sp. other than Mycobacterium avium subsp.
paratuberculosis. FEMS microbiology letters. 2002; 209: 267-271.
- Rachlin J, Ding C, Cantor C, Kasif S. Computational tradeoffs in multiplex PCR assay design for SNP genotyping. BMC genomics. 2005;
6: 1-1.
- Clark Jr DL, Koziczkowski JJ, Radcliff RP, Carlson RA, Ellingson JL.
Detection of Mycobacterium avium subspecies paratuberculosis:
comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. Journal of
dairy science. 2008; 91: 2620-2627.
- Bannantine JP, Baechler E, Zhang Q, Li L, Kapur V. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis
with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. Journal of clinical microbiology. 2002; 40: 1303-1310.
- Rajeev S, Zhang Y, Sreevatsan S, Motiwala AS, Byrum B. Evaluation
of multiple genomic targets for identification and confirmation of
Mycobacterium avium subsp. paratuberculosis isolates using real-time PCR. Veterinary microbiology. 2005; 105: 215-221.
- Stabel JR, Bannantine JP. Development of a nested PCR method
targeting a unique multicopy element, ISMap 02, for detection of
Mycobacterium avium subsp. paratuberculosis in fecal samples.
Journal of Clinical Microbiology. 2005; 43: 4744-4750.
- Maroudam V, Mohana Subramanian B, Praveen Kumar P, Dhinakar
Raj G. Paratuberculosis: diagnostic methods and their constraints.
J Veterinar Sci Technol. 2015; 6: 1000259.
- Jungersen G, Mikkelsen H, Grell SN. Use of the johnin PPD interferon-gamma assay in control of bovine paratuberculosis. Veterinary
immunology and immunopathology. 2012; 148: 48-54.
- Holbert S, Branger M, Souriau A, Lamoureux B, Ganneau C, et al.
Interferon gamma response to Mycobacterium avium subsp. paratuberculosis specific lipopentapeptide antigen L5P in cattle. Research in Veterinary Science. 2015; 102: 118-1121.
- Collins MT, Wells SJ, Petrini KR, Collins JE, Schultz RD, Whitlock RH.
Evaluation of five antibody detection tests for diagnosis of bovine
paratuberculosis. Clinical and Vaccine Immunology. 2005; 12: 685-692.
- Collins MT. Diagnosis of paratuberculosis. Veterinary Clinics of
North America: Food Animal Practice. 1996; 12: 357-371.
- Carter MA. Prevalence and prevention of paratuberculosis in
North America. Japanese Journal of Veterinary Research. 2012;
60: S9-18.
- Bastida F, Juste RA. Paratuberculosis control: a review with a focus
on vaccination. Journal of immune based therapies and vaccines.
2011; 9: 1-7.
- Hines ME, Turnquist SE, Ilha MR, Rajeev S, Jones AL, et al. Evaluation of novel oral vaccine candidates and validation of a caprine
model of Johne’s disease. Frontiers in cellular and infection microbiology. 2014; 4: 26.
- Bull TJ, Schock A, Sharp JM, Greene M, McKendrick IJ, et al. Genomic variations associated with attenuation in Mycobacterium
avium subsp. paratuberculosisvaccine strains. Bmc Microbiology.
2013; 13: 1-7.
- Kwong GP, Poljak Z, Deardon R, Dewey CE. Bayesian analysis of risk
factors for infection with a genotype of porcine reproductive and
respiratory syndrome virus in Ontario swine herds using monitoring data. Preventive veterinary medicine. 2013; 110: 405-417.
- Walker KB, Brennan MJ, Ho MM, Eskola J, Thiry G, et al. The second
Geneva Consensus: Recommendations for novel live TB vaccines.
Vaccine. 2010; 28: 2259-2270.
- Hines II ME, Stiver S, Giri D, Whittington L, Watson C, et al. Efficacy
of spheroplastic and cell-wall competent vaccines for Mycobacterium avium subsp. paratuberculosis in experimentally-challenged
baby goats. Veterinary microbiology. 2007; 120: 261-283.
- Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell host & microbe. 2013; 13: 250-262.
Everman JL, Bermudez LE. Antibodies against invasive phenotype-specific antigens increase Mycobacterium avium subspecies paratuberculosis translocation across a polarized epithelial cell model
and enhance killing by bovine macrophages. Frontiers in cellular
and infection microbiology. 2015; 5: 58.
- Borody TJ, Leis S, Warren EF, Surace R. Treatment of severe Crohn’s
disease using antimycobacterial triple therapy—approaching a
cure?. Digestive and Liver Disease. 2002; 34: 29-38.
- Marcus GF, Davis E. Still searching for principles: A response to
Goodman et al. (2015). Psychological Science. 2015; 26: 542-544.
- Singh JS, Koushal S, Kumar A, Vimal SR, Gupta VK. Book review:
microbial inoculants in sustainable agricultural productivity-Vol. II:
functional application.
- Warrier PK, Nambiar VP, Ramankutty C. Indian Medicinal Plants,
Orient Longman. Chennai (India). 1995.
- Kothari SK, Bhattacharya AK, Ramesh S, Garg SN, Khanuja SP.
Volatile constituents in oil from different plant parts of methyl
eugenol-rich Ocimum tenuiflorum Lf (syn. O. sanctum L.) grown in
South India. Journal of Essential Oil Research. 2005; 17: 656-658.
- Bast F, Rani P, Meena D. Chloroplast DNA phylogeography of holy
basil (Ocimum tenuiflorum) in Indian subcontinent. The Scientific
World Journal. 2014; 2014.
- Wagner RK, Torgesen JK, Rashotte CA. Development of reading-related phonological processing abilities: New evidence of bidirectional causality from a latent variable longitudinal study. Develop-
mental psychology. 1994; 30: 73.
- Mandal DK, Kishore N, Brewer CF. Thermodynamics of lectin-carbohydrate interactions. Titration microcalorimetry measurements
of the binding of N-linked carbohydrates and ovalbumin to concanavalin A. Biochemistry. 1994; 33: 1149-1156.
- Sembulingam K, Sembulingam P, Namasivayam A. Effect of Ocimum sanctum Linn on noise induced changes in plasma corticosterone level. Indian Journal of Physiology and Pharmacology. 1997;
41: 139-143.
- Banu GS, Kumar G, Murugesan AG. RETRACTED: Effects of leaves
extract of Ocimum sanctum L. on arsenic-induced toxicity in Wistar albino rats.
- Godhwani S, Godhwani JL, Vyas DS. Ocimum sanctum: an experimental study evaluating its anti-inflammatory, analgesic and antipyretic activity in animals. Journal of Ethnopharmacology. 1987;
21: 153-163.
- Maity A, Pore N, Lee J, Solomon D, O’Rourke DM. Epidermal growth
factor receptor transcriptionally up-regulates vascular endothelial
growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that
induced by hypoxia. Cancer research. 2000; 60: 5879-5886.
- Agrawal R, Mehta M, Shafer JC, Srikant R, Arning A, Bollinger T. The
Quest Data Mining System. InKDD. 1996; 96: 244-249.
- Joshi CG, Magar NG. Antibiotic activity of some Indian medicinal
plants. J Sci Ind Res. 1952; 11: 261.
- Kapoor LD. CRC handbook of Ayurvedic medicinal plants. CRC
press. 2018.
- Ahmad A, Wani MY, Khan A, Manzoor N, Molepo J. Synergistic interactions of eugenol-tosylate and its congeners with fluconazole
against Candida albicans. Plos one. 2015; 10: e0145053.
- Federman C, Ma C, Biswas D. Major components of orange oil inhibit Staphylococcus aureus growth and biofilm formation, and alter its virulence factors. Journal of medical microbiology. 2016; 65:
688-695.
- Do Nascimento PG, Lemos TL, Bizerra AM, Arriaga ÂM, Ferreira
DA, et al. Antibacterial and antioxidant activities of ursolic acid and
derivatives. Molecules. 2014; 19: 1317-1327.
- Santos EL, Freitas PR, Araújo AC, Almeida RS, Tintino SR, et al.
Enhanced antibacterial effect of antibiotics by the essential oil of
Aloysia gratissima (Gillies & Hook.) Tronc. and its major constituent beta-caryophyllene. Phytomedicine Plus. 2021; 1: 100100.
- Haque MN, Chowdhury R, Islam KM, Akbar MA. Propionic acid is
an alternative to antibiotics in poultry diet. Bangladesh Journal of
Animal Science. 2009; 38: 115-122.
- Abedini A, Roumy V, Mahieux S, Biabiany M, Standaert-Vitse A, et
al. Rosmarinic acid and its methyl ester as antimicrobial components of the hydromethanolic extract of Hyptis atrorubens Poit.
(Lamiaceae). Evidence-Based Complementary and Alternative
Medicine. 2013; 2013.
- Nayaka HB, Londonkar RL, Umesh MK, Tukappa A. Antibacterial
attributes of apigenin, isolated from Portulaca oleracea L. International journal of bacteriology. 2014; 2014.
- Karpiński T, Adamczak A, Ożarowski M. Antibacterial activity of apigenin, luteolin, and their C-glucosides. InProceedings of the 5th
International Electronic Conference on Medicinal Chemistry. 2019.
- https://www.chemfaces.com/natural/Isothymusin-CFN97562.
html.
- Mohotti S, Rajendran S, Muhammad T, Strömstedt AA, Adhikari A,
et al. Screening for bioactive secondary metabolites in Sri Lankan
medicinal plants by microfractionation and targeted isolation of
antimicrobial flavonoids from Derris scandens. Journal of Ethnopharmacology. 2020; 246: 112158.
- Singh OM, Singh TP. Phytochemistry of Solanum xanthocarpum: an
amazing traditional healer.
- Hussain T, Gupta RK, Sweety K, Khan MS, Hussain MS, et al. Studies on hypoglycaemic activity of Solanum xanthocarpum Schrad. &
Wendl. fruit extract in rats. Journal of Ethnopharmacology. 2006;
108: 251-256.
- Kirtikar KR, Basu BD. Indian medicinal plants. Indian Medicinal
Plants.1918.
- Kumar S, Pandey AK. Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: an in
vitro antioxidant, anticancer and anti HIV perspective. BMC Complementary and Alternative Medicine. 2014; 14: 1-8.
- Ayurvedic Pharmacopoeia Committee. The ayurvedic pharmacopoeia of India. Government of India, Ministry of Health and Family
Welfare. New Delhi, India: Department of AYUSH. 2001.
- Hussain T, Gupta RK, Sweety K, Khan MS, Hussain MS, Arif MD,
Hussain A, Faiyazuddin MD, Rao CV. Evaluation of antihepatotoxic
potential of Solanum xanthocarpum fruit extract against antitubercular drugs induced hepatopathy in experimental rodents.
Asian Pacific journal of tropical biomedicine. 2012; 2: 454-460.
- Lou Z, Wang H, Zhu S, Ma C, Wang Z. Antibacterial activity and
mechanism of action of chlorogenic acid. Journal of food science.
2011; 76: M398-403.
- Swain SS, Padhy RN. In vitro antibacterial efficacy of plants used
by an Indian aboriginal tribe against pathogenic bacteria isolated
from clinical samples. Journal of Taibah University Medical Sciences. 2015; 10: 379-390.
- Huang ZH, Hua D, Du X. Polymorphisms in p53, GSTP1 and XRCC1
predict relapse and survival of gastric cancer patients treated with
oxaliplatin-based adjuvant chemotherapy. Cancer chemotherapy
and pharmacology. 2009; 64: 1001-1007.
- Al Sinani SS, Eltayeb EA. The steroidal glycoalkaloids solamargine
and solasonine in Solanum plants. South African Journal of Botany.
2017; 112: 253-269.
- Kumar P, Sharma B, Bakshi N. Biological activity of alkaloids from
Solanum dulcamara L. Natural product research. 2009; 23: 719-723.
- Woldemichael GM, Gutierrez-Lugo MT, Franzblau SG, Wang Y, Suarez E, et al. Mycobacterium t uberculosis Growth Inhibition by
Constituents of Sapium haematospermum. Journal of Natural
products. 2004; 67: 598-603.
- Mailafiya MM, Yusuf AJ, Abdullahi MI, Aleku GA, Ibrahim IA, et al.
Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. Journal of Medicinal Plants for Economic Development. 2018; 2: 1-5.
- Nweze C, Ibrahim H, Ndukwe GI. Beta-sitosterol with antimicrobial
property from the stem bark of pomegranate (Punica granatum
Linn). Journal of Applied Sciences and Environmental Management. 2019; 23: 1045-1049.
- Nayaka HB, Londonkar RL, Umesh MK, Tukappa A. Antibacterial
attributes of apigenin, isolated from Portulaca oleracea L. International journal of bacteriology. 2014; 2014.
- Pushpanathan M, Gunasekaran P, Rajendhran J. Antimicrobial peptides: versatile biological properties. International journal of peptides. 2013; 2013.
- Mokdad-Bzeouich I, Mustapha N, Chaabane F, Ghedira Z, Ghedira
K, et al. Oligomerization of esculin improves its antibacterial activity and modulates antibiotic resistance. The Journal of antibiotics.
2015; 68: 148-152.
- Lemos AS, Florêncio JR, Pinto NC, Campos LM, Silva TP, et al. Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Frontiers in Microbiology. 2020; 11: 1525.