Introduction
Widespread use of mammography as a screening tool has resulted in increasing numbers of breast biopsies performed for
subclinical mammographic abnormalities (microcalcifications, abnormalities in density or opacities, etc). On these biopsies, surgical pathologists frequently encounter lesions designated as Flat
Epithelial Atypia (FEA), a term introduced by the World Health
Organization (WHO) Working Group on the Pathology and Genetics of Tumors of the Breast. Historically, FEA has been described
variably, but in 2019, FEA was defined by the WHO as Terminal
Duct Lobular Units (TDLUs) with enlarged dilated acini with morerounded contours; lined by one to several layers of mildly atypical
cuboidal to columnar cells resembling the monomorphic nuclei of
low-grade Ductal Carcinoma in Situ (DCIS) [1].
The association of atypical hyperplasia which includes both
Atypical Ductal Hyperplasia (ADH) and Atypical Lobular Hyperplasia (ALH), with an increased risk of invasive breast carcinoma has
been well established. A study concludes that atypical hyperplasia
confers an absolute risk of subsequent breast cancer of 30% at
25 years of follow-up [2]. However, the management of FEA diagnosed on Core Needle Biopsy (CNB) varies between institutions,
largely due to uncertainty of its biologic potential and its association with more advanced lesions. Excision versus observation with
radiological follow-up for FEA remains controversial. In this study,
we attempted to determine the clinical significance of FEA on CNB
based on the upgrade on subsequent excisions, using a single institution experience.
Materials and methods
All material to be used for the study were collected from the
archives of the pathology department and were previously formalin fixed and paraffin embedded. Retrospective histopathologic
review was performed on CNB with diagnosis of pure FEA for a
period of 11 years (January 2010-December 2021) (According to
the introduction, FEA has been described variably, but in 2019,
our institute descript FEA since 2018) and corresponding subsequent excisions. Cases with co-existing Atypical Ductal Hyperplasia (ADH) or more advanced lesions (ductal carcinoma in-situ or
invasive mammary carcinoma) within the same biopsy cores were
excluded from the study. Cases with FEA on CNB without subsequent excisions were also excluded. Institutional ethical approval
was obtained for this study, which did not require informed consent.
Results
The pathology reports of thirty (30) cases with pure FEA on
CNB and subsequent excisions were retrospectively reviewed.
The mean age of this patient group was 48.9 years. The 73.3%
of cases (22/30) did not show an upgrade in diagnosis on excision. The remaining 8/30 cases (26.7%) showed an upgrade of FEA
diagnosis on subsequent excisions, including 6/30 cases (20%)
with co-existing diagnosis of ADH, and 2/30 cases (6.6%) with coexisting diagnosis of either Markedly ADH bordering on Ductal
Carcinoma In-Situ (MADH/DCIS), or DCIS. Lobular Intraepithelial
Neoplasia (LIN) including Atypical Lobular Hyperplasia (ALH) or
Lobular Carcinoma In-Situ (LCIS) were seen in 11/30 (36.6%) in
association with FEA.
Discussion
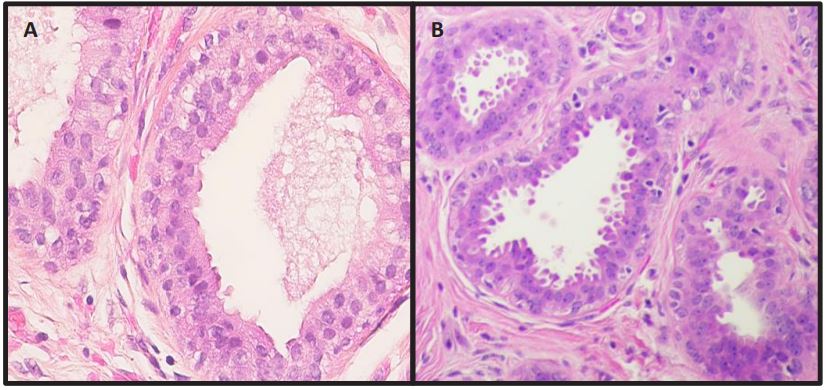
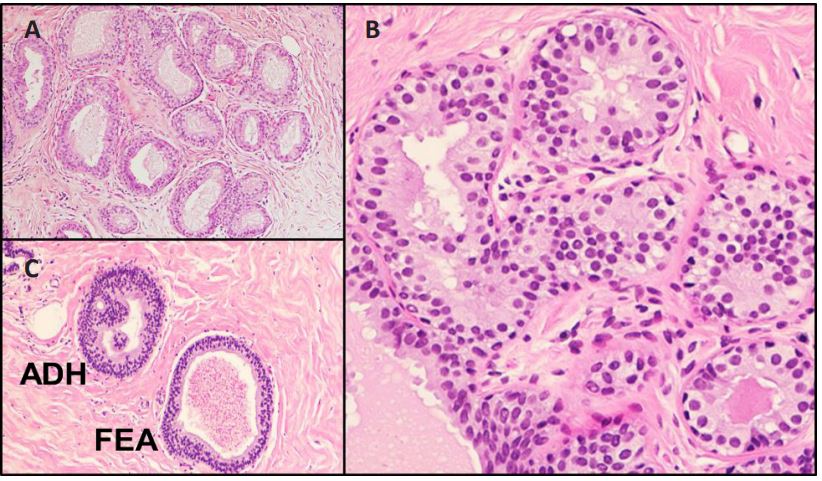
FEA is characterized by the dilated ducts lined by 1 or 2 to 3 layers of atypical cuboidal or columnar cells (Figure 1) and absence
of any architectural complexity, such as focal trabeculae, Roman
arches, micropapillae, and cribriforming (Figure 2). The cells retain the apical snouts and may be associated with calcifications.
FEA is increasingly found on CNB. In the literature, the lowest reported prevalence is 1.5 %, going higher to 3.7 %, with the highest
reported prevalence of 35.2 % [3-13]. Emerging data suggest that
FEA most likely represents the earliest morphologically recognizable precursor of low-grade DCIS [14]. The clinical significance of
this entity has been hampered by variation in terminology, diagnostic challenge to surgical pathologists, as well as the limited
number of cases that have been studied in a systematic fashion
[15]. Studies to assess reproducibility in the evaluation of FEA
have demonstrated only moderate interobserver reproducibility
after tutorial among surgical pathologists [16].
Most studies in the literature have recorded/monitored the
outcome of a spectrum of atypical lesions including FEA with
other forms of atypia (ADH, ALH or LN). Scattered case reports
along with few recent papers analyzing pooled data have looked
at outcome of isolated FEA without other forms of atypia on CNB.
Available evidence regarding the clinical significance of FEA from
the limited number of formerly published series is widely varied.
Various upgrade results with recommended management plans
were proposed by authors accordingly (refer to Table 1 summarization). There are reports that no upgraded cases were found in
subsequent excision in the patients with pure FEA on CNB [15,17], therefore, the authors propose that in cases with FEA not associated with other atypia could be spared surgical excision and
managed with close radiologic follow-up. However, there are also
studies in the literature reported the upgrade rate of FEA on CNB
in the subsequent excision varies from 6.7 % to 25 % [3-12], hence
surgical excision is the favored management.
No radiologic features are diagnostic of FEA, but usually FEA
presents as an area of mammographic calcifications. One study
examined the upgrade rate following non-surgical management
of patients who had a biopsy with FEA which targeted microcalcifications, completely removed on biopsy. Only one of 48 patients
(2%) who had new microcalcifications developed 26 months after prior biopsy yielded upgraded diagnosis to ADH. Therefore,
the author proposed that surgical excision may not be necessary
for pure FEA diagnosed on CNB if targeted microcalcifications are
largely removed during the biopsy procedure and no residual microcalcifications are present immediate after the biopsy or on the
follow-up radiologic evaluation [20].
Our results showed 26.7% upgraded cases of pure FEA to either ADH or in situ carcinoma. This is consistent with the results
of two most recent systematic review with meta-analysis and
largest series [18,19]. Overall, therefore, our findings support
surgical excision when FEA is diagnosed on CNB. Our study, however, has several limitations. First, the study was retrospective.
Second, the sample size is relatively small. Third, our study was not multi-institutional. Fourth, FEA diagnosis was made by various pathologists, and information about inter observer variability
among pathologists was not obtained. Inter observer variability
even among breast pathologists is known in the diagnosis of FEA
in spite of published guidelines as mentioned earlier. Lastly assessing for residual lesion after CNB was difficult due to absence
of specimen post-biopsy radiograph assessment. Therefore, this
decision should be taken multidisciplinary by radiologists, pathologists, and surgeons.
Conflict of interest: Authors do not have any conflict of interest.
References
- Tan PH, Ellis I, Allison K, Brogi E, Fox SB, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020; 77(2): 181-185. doi: 10.1111/his.14091.
- Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast-risk assessment and management options. N Engl J Med. 2015; 372(1): 78-89. doi: 10.1056/NEJMsr1407164.
- Noske A, Pahl S, Fallenberg E, Richter-Ehrenstein C, BuckendahlAC, et al. Flat epithelial atypia is a common subtype of B3 breast lesions and is associated with noninvasive cancer but not with invasive cancer in final excision histology. Human Pathol. 2010; 41: 522-7.
- De Mascarel I, MacGrogan G, Mathoulin-Pe´lissier S, Vincent- Salomon A, Soubeyran I, et al. Epithelial atypia in biopsies performed for microcalcifications. Practical considerations about 2,833 serially sectioned surgical biopsies with a long follow-up. Virchows Arch. 2007; 451: 1-10.
- Guerra-Wallace MM, Christensen WN, White RL. A retrospective study of columnar alteration with prominent apical snouts and secretions and the association with cancer. Am J Surg. 2004; 188: 395-8.
- Lavoue´ V, Roger CM, Poilblanc M, Proust N, Monghal-Verge C, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: Study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat. 2011; 1-6.
- Chivukula M, Bhargava R, Tseng G, Dabbs DJ. Clinicopathologic implications of ‘‘flat epithelial atypia’’ in core needle biopsy specimens of the breast. Am J Clin Pathol. 2009; 131: 802.
- Lee TYJ, MacIntosh RF, Rayson D, Barnes PJ. Flat epithelial atypia on breast needle core biopsy: A retrospective study with clinicalpathological correlation. Breast J. 2010; 16: 377-83.
- Solorzano S, Mesurolle B, Omeroglu A, El Khoury M, Kao E, et al. Flat epithelial atypia of the breast: Pathological radiological correlation. Am J Roentgenol. 2011; 197: 740-6.
- David N, Labbe-Devilliers C, Moreau D, Loussouarn D, Campion L. Diagnosis of Flat Epithelial Atypia (FEA) after stereotactic VacuumAssisted Biopsy (VAB) of the breast: What is the best management: Systematic surgery for all or follow-up?. J Radiologie. 2006; 87(11): 1671.
- Ingegnoli A, D’Aloia C, Frattaruolo A, Pallavera L, Martella E, et al. Flat epithelial atypia and atypical ductal hyperplasia: Carcinoma underestimation rate. Breast J. 2010; 16: 55-9.
- Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: Should it be excised? Human Pathol. 2007; 38: 35-41.
- Martel M, Barron-Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992-1999). Virchows Arch. 2007; 451: 883-91.
- Schnitt SJ, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol. 2003; 10: 113-24.
- Senetta R, Campanino PP, Mariscotti G, Garberoglio S, Daniele L, et al. Columnar cell lesions associated with breast calcifications on vacuum-assisted core biopsies: Clinical, radiographic, and histological correlations. Mod Pathol. 2009; 22: 762-9.
- Tan PH, Ho BC, Selvarajan S, Yap WM, Hanby A. Pathological diagnosis of columnar cell lesions of the breast: Are there issues of reproducibility? J Clin Pathol. 2005; 58: 705- 9.
- Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, et al. Flat epithelial atypia on core needle biopsy: Which is the right management? Am J Surg Pathol. 2009; 33: 1078.
- Rudin AV, Hoskin TL, Fahy A, Farrell AM, Nassar A, et al. Flat Epithelial Atypia on Core Biopsy and Upgrade to Cancer: a Systematic Review and Meta-Analysis. Ann Surg Oncol. 2017; 24(12): 3549-3558.
- Wahab RA, Lee SJ, Mulligan ME, Zhang B, Mahoney MC. Upgrade Rate of Pure Flat Epithelial Atypia Diagnosed at Core Needle Biopsy: A Systematic Review and Meta-Analysis. Radiol Imaging Cancer. 2021; 3(1): e200116.
- Schiaffino S, Gristina L, Villa A, et al. Flat epithelial atypia: conservative management of patients without residual microcalcifications post-vacuum-assisted breast biopsy. Br J Radiol. 2018; 91(1081): 20170484.
- Grabenstetter A, Brennan S, Salagean ED, Morrow M, Brogi E. Flat Epithelial Atypia in Breast Core Needle Biopsies With RadiologicPathologic Concordance: Is Excision Necessary? Am J Surg Pathol. 2020; 44(2): 182-190.
- Liu C, Dingee CK, Warburton R, Pao JS, Kuusk U, et al. Pure flat epithelial atypia identified on core needle biopsy does not require excision. Eur J Surg Oncol. 2020; 46(2): 235-239.
- Lavoué V, Roger CM, Poilblanc M, Proust N, Monghal-Verge C, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat. 2011; 125(1): 121-6.
- Khoumais NA, Scaranelo AM, Moshonov H, Kulkarni SR, Miller N, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol. 2013; 20(1): 133-8.
- Noël JC, Buxant F, Engohan-Aloghe C. Immediate surgical resection of residual microcalcifications after a diagnosis of pure flat epithelial atypia on core biopsy: A word of caution. Surg Oncol. 2010; 19(4): 243-6.