Introduction
Thyroid Cancer (TC) has experienced a rise in incidence since
the early 1980s and now ranks as the fifth most common cancer among women in the United States. It is projected to become
the most commonly diagnosed cancer in people aged 15 to 29
and the fastest-growing cancer in many countries, largely due to
an increase in Papillary Thyroid Cancer (PTC) [1-3]. The Follicular
Variant of Papillary Thyroid Carcinoma (FVPTC) is the second most
common histological subtype within PTC, accounting for 9-27% of
all PTC patients [4-6].
FVPTCs are classified into two subgroups: encapsulated and
infiltrative forms [7]. Encapsulated EFVPTC is further divided into
non-invasive EFVPTC and invasive EFVPTC, based on the invasion
of the capsule by tumor cells [8]. Overall, non-invasive EFVPTC has
been found to exhibit a less aggressive recurrence and metastasis
rate than other PTC variants [9,10]. In 2016, Nikiforov et al. [11]
suggested renaming non-invasive EFVPTC to exclude the word
“carcinoma” from its nomenclature and introduced the term
“Non-Invasive Follicular Thyroid Neoplasm with Papillary-Like
Nuclear Features” (NIFTP). The 2017 World Health Organization
classification of neoplasms removed NIFTP from the list of cancers, emphasizing its favorable treatment outcomes and limited
malignant potential, which result in a mild course of the disease
during follow-up [12].
Somatic mutational profiling has identified driver mutations
that are believed to contribute to early carcinogenesis, diagnosis,
and therapy [13]. In recent decades, research on human cancer
genetics has greatly benefitted from new technologies such as
Sanger sequencing, Polymerase Chain Reaction (PCR), and NextGeneration Sequencing (NGS) approaches. These include WholeGenome Sequencing (WGS), whole-exome sequencing (WES), and
targeted panels, which have identified mutations with prognostic
significance [14-17].
Recent large-scale whole-genome and whole-exome sequencing studies have aimed to identify the genetic causes of FVPTC,
with varying degrees of success. In FVPTC, one of the most common genetic alterations is Rat Sarcoma viral oncogene (RAS) mutations, which is a key protein in many signaling pathways that
regulates normal cell growth and malignant transformation and
occurs at a frequency of 15-40% [18-20]. V-raf murine sarcoma
viral oncogene homolog B(BRAF) is a serine/threonine protein kinase activated by the Ras-GTP protein [21]. The most frequent
BRAF mutation in FVPTC is the T1799A transversion mutation in
exon 15 of the gene, which causes a V600E amino acid substitution in the protein [22]. Additionally, Telomerase Reverse Transcriptase (TERT) mutations occur in two hotspot positions located
124 and 146 bp upstream from the ATG start site (124 G4A and 146
G4A, C4T on opposite strand), enhancing TERT promoter activity
[23]. The Cancer Genome Atlas identified additional driver alterations present at a lower frequency, including EIF1AX, PPM1D, and
CHEK2 [24]. The presence or absence of each of these genetic
markers may have therapeutic and/or prognostic implications for
patients with FVPTC.
The frequency of somatic mutations in FVPTC varies considerably, and many literature studies have reported molecular abnormalities in FVPTC. While BRAF mutations have been shown to
have a strong positive correlation with poor clinical characteristics of FVPTC [25-28], others found no such connection [29-32].
Similarly, RAS mutations in FVPTC have yielded contradictory outcomes [16,33-37]. Additionally, a wide range of mutation landscapes has been observed for other mutations due to objective
factors such as sample size, ethnicity, and mutation analysis methodologies [23,38-41]. However, a comprehensive or pooled metaanalysis of the entire somatic mutational landscape of FVPTC is
currently lacking.
Here, we present a meta-analysis of the somatic mutation
landscape of FVPTC, assessing the prevalence and clinical characteristics of these mutations. The analysis includes ethnicity, tumor
preservation conditions, gene sequencing methods, and reference quality to demonstrate the potential clinical significance of
these mutations.
Materials and methods
This systematic review was conducted following the methods
outlined in the Preferred Reporting Items for Systematic Review
and Meta-Analyses (PRISMA) guidelines [42].
Search strategy
To conduct this research, we selected articles from the
Pubmed/MEDLINE, Embase, and The Cancer Genome Atlas databases between January 2013 and December 2023. Our search included both indexing terms (MeSH terms in PubMed and ENTREE
terms in Embase) and keyword terms such as “Genomic [Mesh]”
or “Mutation” and (“Follicular Variant of Papillary Thyroid Carcinoma” or “Encapsulated Follicular Variant of Papillary Thyroid
Carcinoma” or “Invasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma” or “Non-Invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features” or Infiltrative Follicular
Variant of Papillary Thyroid Carcinoma” or “FVPTC” or “EFVPTC”
or “IEFVPTC” or “NIFTP” or “IFVPTC”). We manually searched the
reference lists of all included articles to identify any potentially
related studies, and used EndNote software to manage references
and remove duplicates. Furthermore, we reviewed the references
cited in the searched articles and relevant studies to ensure that
no eligible articles were missed.
Eligibility criteria
Our objective was to conduct a meta-analysis on genomic data
obtained from FVPTCs to assess the prevalence of gene mutations
and clinical features. We used a modified PICOS (participants, interventions, comparators, outcomes, and studies) approach to
guide our screening of studies for eligibility in our analysis.
Details of the inclusion and exclusion criteria can be found in
the Supplementary methods [43].
Study selection
Two authors (Fan and Zhang), screened the retrieved papers
independently. They first screened them by title, then by abstract,
and finally by full text. Any disagreements during screening were resolved through discussion and consensus. In cases where disagreements persisted, a third researcher (Huang) was consulted.
The following information was extracted from each study using a
predefined worksheet: title, journal, publication year, study design, country, institution, time of enrollment, sequencing method
used, type of FVPTC, and mutational genes involved. If required,
the authors of each trial were contacted for additional information.
Quality assessment
Qgenie-tool was used to perform a literature quality assessment for all included articles [44].
Statistical analysis
The prevalence of somatic mutations, including point mutations, was presented using forest plots with 95% confidence intervals in R Studio version 1.3 and the “Meta” package. Heterogeneity was assessed using x2
-based Q statistics and I2
, where P
values <0.05 for the Cochran Q test and I2
exceeding 50% were
considered significant. Publication bias was evaluated using funnel plot of standard error against the effect estimate, and statistical significance was determined by a P value <0.05 using the Egger
linear regression test method. Subgroup analyses were conducted for FVPTC subtypes, tumor preservation conditions, ethnicity,
gene test method, Q-genie quality score, and research center. The
Z-test was used to evaluate differences between pooled proportions of prevalence, and statistical significance was set at P<0.05.
The “maftools” R package [45] was used to visualize oncoplot, somatic interaction, and position-based cancer driver analytics, and
to calculate the number of somatic non-synonymous point mutations within each sample.
Results
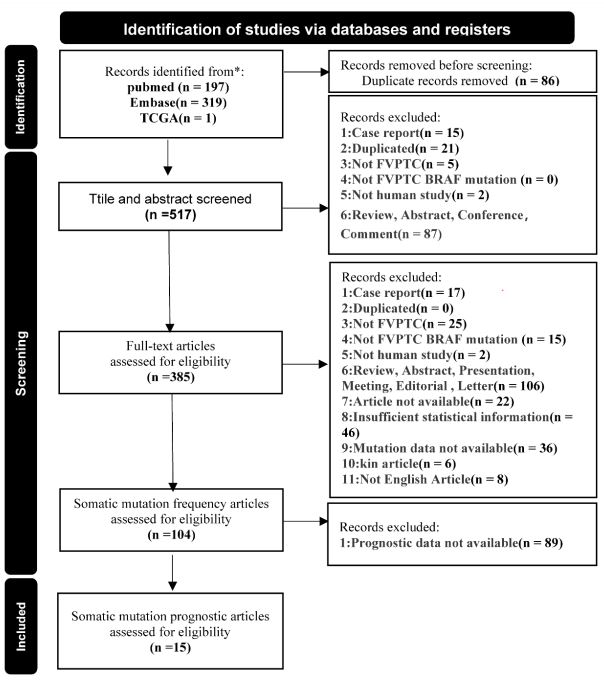
Eligible studies: After conducting our initial literature search
and removed 86 duplicates, we found a total of 197 relevant
abstracts on PubMed, 319 on Embase, and an additional record
from the TCGA database (Figure 1). Following the elimination of
duplicates and screening of titles and abstracts, we were left with
130 publications. After a careful review of full-text articles, 281
irrelevant records were removed, leaving us with 104 articles for
frequency meta-analysis. Unfortunately, due to a lack of available
clinical data, 89 papers were excluded. Ultimately, our clinical feature-related meta-analysis was based on 15 independent studies,
in addition to mutation data from the TCGA database.
Study characteristics: In summary, publication details for the
studies are provided in the supplementary reference list in the
Supplementary Table S1 [43]. Our meta-analysis included a total
of 7971 FVPTC patients, with the majority being women. The genotype data were primarily obtained from Formalin-Fixed ParaffinEmbedding samples (FFPE) from patients, and direct sequencing
was the most common method used. Our analysis consisted of
92 single-center studies and 12 multi-center studies, which are
described in Table S1 [43] along with their basic features and enrollment details. And 31 high-quality studies were identified, as
shown in Table S2 [43].
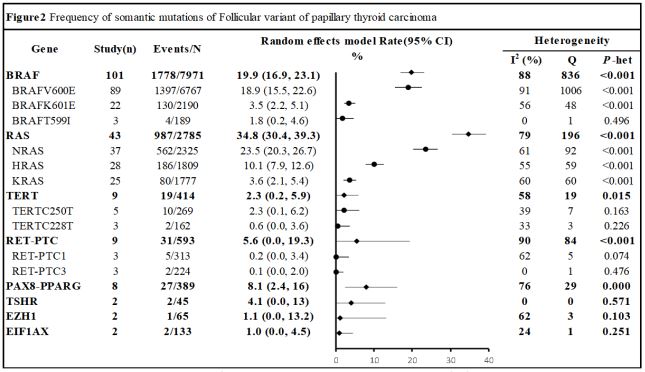
Somatic mutation frequencies of FVPTC: Our frequency metaanalysis included a total of 104 studies. We selected 35 mutated
genes using a priori mutation prevalence threshold of 1%.
In our pooled meta-analysis of FVPTCs, we grouped mutations
and compared them with total mutations and high mutational
points. As shown in Figure 2, the prevalence of somatic mutations ranged from 1% to 35% in FVPTCs. The most commonly
mutated gene was RAS (34.8%; 95%CI, 30.4%-39.3%), which had
three main mutational sites: NRAS (23.5%; 95% CI, 20.3%-26.7%),
HRAS (10.1%; 95% CI, 7.9%-12.6%), and KRAS (3.6%; 95% CI, 2.1%-
5.4%). The other four most frequently studied genes were BRAF,
RET-PTC, TERT, and PAX8-PPARG, with mutation prevalences of
19.9%, 5.6%, 2.3%, and 8.1%, respectively. Notably, parts of the
mutation gene (THDA1, TET2, SMARCB1, etc) only having one to
three articles described, were summarized in Table S3 [43].
Generally, our pooled meta-analysis involved a diverse range
of research studies, leading to significant heterogeneity. Therefore, we focused our subgroup analysis on RAS, BRAF, TERT, RETPTC, and PAX8-PPARG. We aimed to identify the reasons for this
heterogeneity by examining various parameters including tumor
preservation conditions (formalin-fixed vs Fresh-FNA), ethnicity
(Western vs Asian), gene test method (Direct Sequencing vs Immunohistochemistry vs Sanger sequencing vs Next-Generation
Sequencing), Q-genie quality score (High vs other), and research
center (single vs multiple centers). Furthermore, we observed a
diverse mutation landscape in other mutations, which could be
attributed to several objective factors such as sample size, ethnicity, and mutation analysis methods.
Table S4 [43]shows that FFPE is the primary preservation method for mutation testing samples. However, there is no significant
difference in mutation frequency between FFPE and FNA samples. Furthermore, ethnicity subgroup analyses revealed similar
population-level differences for BRAF and RAS mutations. Direct
sequencing was the most commonly used test for these two mutations, with a BRAF mutation frequency of 17.2% in Westerners
compared to 24.4% in Easterners and 21.7% by direct sequencing. In contrast, the multi-center study reported a TERT mutation
frequency of 10.6%, which was significantly higher than the 1.8%
reported in the uni-center study. Lastly, there was no significant
difference in mutation frequency between the two levels scores.
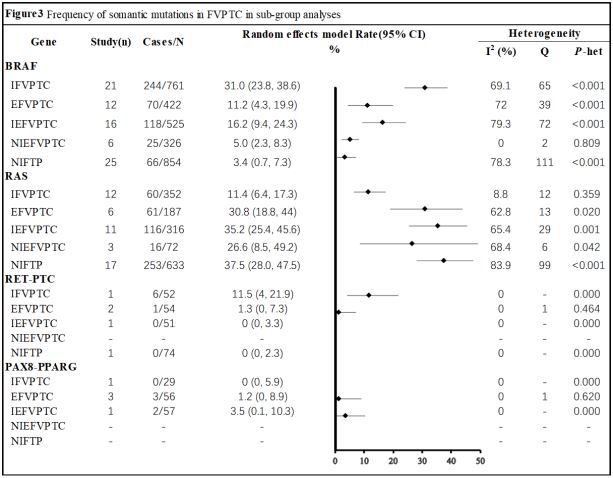
Prevalence of individual mutations by histology: Interestingly,
the RAS gene (rate 37.5%; 95% CI, 28.0%-47.5%) had the highest occurrence of mutations among all genes listed in Figure 3.
The frequency was significantly greater in the NIFTP subgroup
compared to other histology groups. Similarly, BRAF mutations
(rate 31.0%; 95% CI, 23.8%-38.6%) were relatively common in
the IFVPTC subgroup. However, IFVPTC showed only about a 10%
prevalence in RAS mutations. Additionally, the prevalence of BRAF
mutations was relatively low in NIFTP (rate 3.4%; 95% CI, 0.7%-
7.3%) and NIEFVPTC (rate 5.0%; 95% CI, 2.3%-8.3%).
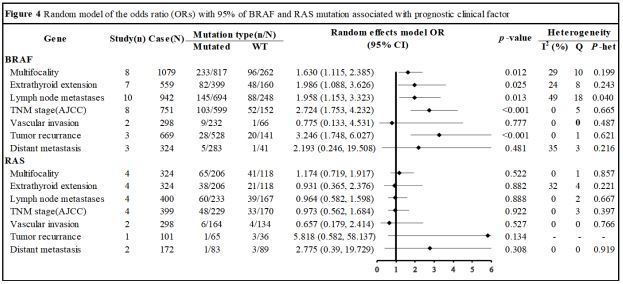
Association between mutations and FVPTCs’ clinical feature: The basic characteristics of the clinical feature-related eligible
studies are summarized in Table S5 [43]. Fourteen and four articles respectively dealt with clinical features associated with BRAF
and RAS mutations.
The BRAF and RAS mutations were characterized in eight and
four trials, respectively, with a total of 1079 and 324 patients.
The BRAF mutation was discovered in 262 patients with a positive mutational status, while the RAS mutation was found in 118
patients (Figure 4). In relation to multifocality in FVPTC, the BRAF mutation was linked with an Odds Ratio (OR) of 1.630 (95% Confidence Interval [CI], 1.115-2.385; Z=2.52; P=0.012), whereas there
were no significant differences between RAS mutation groups
(OR, 1.174; 95% CI, 0.719-1.917; Z=0.64; P=0.522). Regarding extrathyroid extension, eight studies involving 559 individuals for
BRAF mutations, and four studies involving 324 individuals for
RAS mutations were discovered, with 160 and 118 patients having a positive mutational status, respectively. The BRAF mutant
was linked with extra thyroid extension (OR, 1.986; 95% CI, 1.088-
3.626; Z=2.23; P=0.025), but not the RAS mutation (OR, 0.931;
95% CI, 0.365-2.376; Z=-0.15; P=0.882). With respect to lymph
node metastases, ten studies encompassing 942 patients for
BRAF mutations and four studies including 400 patients for RAS
mutations were discovered, with 248 and 167 individuals having a positive mutational status, respectively. The BRAF mutation
was related to lymph node metastases (OR, 1.958; 95% CI, 1.153-
3.323; Z=2.49; P=0.013), but there were no significant differences
between RAS mutation groups (OR, 0.964; 95% CI, 0.582-1.598;
Z=-0.14; P=0.888). Eight investigations encompassing 751 patients for BRAF mutations and four studies including 399 patients
for RAS mutations in relation to advanced TNM stage were discovered, with 152 and 170 patients having a positive mutational
status, respectively. The BRAF mutation was related to advanced
TNM stage (OR, 2.724; 95% CI, 1.753-4.232; Z=4.46; P<0.001),
but there were no significant differences between RAS mutation groups (OR, 0.8960; 95% CI, 0.562-1.684; Z=-0.1; P=0.922).
Both BRAF and RAS mutations were found in two investigations
encompassing 298 patients each, in terms of vascular invasion,
with 66 and 134 individuals having a positive mutational status,
respectively. However, neither BRAF nor RAS mutations were significantly related to vascular invasion (OR, 0.775; 95% CI, 0.133-
4.531; Z=-0.28; P=0.777 | OR, 0.6571; 95% CI, 0.1789-2.4138;
Z=2.49; P=0.527). Three studies encompassing 669 patients for
BRAF mutations and one study including 101 patients for RAS mutations in relation to tumor recurrence were found, with 141 and
34 patients having a positive mutational status, respectively. The
BRAF mutation was linked to tumor recurrence (OR, 3.2460; 95%
CI, 1.7481-6.0272; Z=2.49; P=0.0002), but not the RAS mutation
(OR, 5.8182; 95% CI, 0.5823-58.1373; Z=2.49; P=0.1338). Regarding distant metastases, three investigations covering 324 patients
for BRAF mutations and one study including 172 patients for RAS
mutations were discovered, with 41 and 89 patients having a positive mutational status, respectively. However, both BRAF and RAS
mutations were unrelated to vascular invasion (OR, 2.193; 95% CI,
0.246-19.508; Z=0.7; P=0.481 | OR, 2.775; 95% CI, 0.39-19.729;
Z=1.02; P=0.308).
The landscape of somatic mutation in FVPTCs: In the TCGATHCA cohort, a total of 102 FVPTC patients were detected, and
their basic characteristics are summarized in Table S6 [43]. As
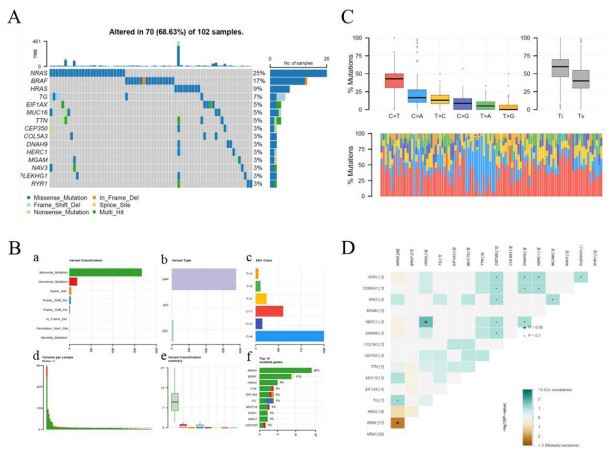
shown in the waterfall map, 70 out of 102 FVPTC patients had
somatic mutations, accounting for 68.63%. The NRAS, BRAF, and
HRAS mutations were the three most highly mutated genes in
FVPTC samples, with frequencies of 25%, 17%, and 9%, respectively (Figure 5A). Missense mutations had an absolute position
among the total mutation classification (Figure 5Ba), and Single
Nucleotide Polymorphisms (SNPs) accounted for a higher proportion than deletions or insertions (Figure 5b and e). Additionally,
C > A had the highest frequency, 1066 times, among the variant
types of SNVs (Figure 5b,c). Figure 5d showed that the number of variants per sample and the median value of mutation variants was 11. Furthermore, the top 10 genetically varied genes in
the TCGA-FVPTC cohort were NRAS, BRAF, HRAS, TTN, EIF1AX, TG,
MUC16, RYR1, NAV3, and CEP350 (Figure 5bf). The distribution
of SNVs in FVPTC was classified into six transition and transversion events, as displayed in the transition and transversion plot
(Figure 5c). The stacked bar plot at the bottom shows the distribution of mutation spectra for every sample in the MAF file. To
further elucidate the intrinsic connection between these genetically altered genes, the exclusive and co-occurrence correlations
were presented in Figure 5D. HRAS and HERC1 had the highest
co-occurrence frequency.
Heterogeneity and publication bias: We performed several
subgroup analyses on the top 5 mutational genes investigated to
understand the variation in mutation prevalence among primary tumors. Unfortunately, our findings were inconsistent (Table S4
[43]), but the complete funnel plots can be viewed in Figure S1
[43]. As per the sensitivity analysis, none of the studies significantly affected the pooled Odds Ratios (ORs) and Confidence Intervals (CIs). Additionally, Figure S2 [43] shows the sensitivity assessment results for the response assessment outcomes.
Discussion
We present a meta-analysis of somatic mutations in FVPTC,
which offers more robust findings for gene mutation prevalence
compared to data from individual studies. Understanding somatic
mutations in FVPTC may aid in categorizing individuals based on
their clinicopathological risk factors. Genes with a higher frequency of mutations ought to be included in future genomic and functional investigations to gain a better understanding of their role in FVPTC, as well as in sequencing panels.
We found a high incidence of RAS mutations (34.8% in patients
at baseline), as shown in Figure 2. These mutations are central to
the development of FVPTC cancer, but they do not appear to be related to the clinical characteristics of malignancy. RAS oncogenes
encode a family of guanine nucleotide-binding proteins and play
a critical role in carcinogenesis and progression [18,19]. As such,
they are considered an important target for therapeutic intervention. The RAS family is composed of three small GTP proteins,
specifically HRAS, NRAS, and KRAS. Studies show that among the
three, NRAS mutations occur more frequently compared to HRAS
mutations. Meanwhile, KRAS mutations are considered rare, accounting for less than 1% of cases [28]. Interestingly, in FVPTC
subtype brackets, we found that RAS mutations were most commonly mutated in NIFTP (Rate, 37.5%; 95% CI, 28.0%-47.5%), but
not in IFVPTC (Rate, 11.4%; 95% CI, 6.4%-17.3%). As Nikiforov et
al. [11] in 2016 suggested, NIFTPs were detected in more than
45,000 patients each year and have a very low risk of adverse outcomes. On the other hand, IFVPTC is more aggressive than both
EFVPTC types for most clinicopathological features [9,25,46]. Although RAS mutations were most commonly found in NIFTP, our
analysis showed no significant association between RAS mutation
and malignancy-related clinical features in FVPTC (Figure 4).
Figure 2 shows that the frequency of BRAF mutations followed
that of RAS mutations in FVPTC. BRAF is one of the three isoforms
of RAF, which has activating missense point mutations clustered
in the kinase domain (exons 11 and 15) [47,48]. The c.T1799A is
the most commonly detected mutation in PTC, resulting in a valine-to-glutamic acid amino acid substitution (BRAFV600E). This
constitutive activation of BRAF kinase may play a role in initiating
tumorigenesis of FVPTC (Figure 5) [49]. Our BRAF mutation results
in different subgroups (Figure 3) indicate that the frequency of
BRAF mutations was lowest in NIFTP (rate: 3.4%; 95% CI: 0.7%-
7.3%), and highest in IFVPTC (rate: 31.0%; 95% CI: 23.8%-38.6%).
The higher the frequency of BRAF mutation, the more aggressive
the histological subtype of FVPTC.
The occurrence and development of FVPTC are also associated with three other mutated genes: TERT, RET-PTC, and PAX8PPARG. Telomerase, a ribonucleoprotein complex that maintains
the length of telomeres at the end of chromosomes, plays a vital
role in cellular immortality and tumorigenesis [50,51]. The C228T
and C250T TERT promoter mutations were detected in follicularderived thyroid cancers, but they were not present in benign or
medullary thyroid cancers [23,52]. Translocation t(2;3) (q13;p25)
that causes the fusion of the DNA-binding domain of the thyroid
transcription factor PAX8 to domains A to F of the peroxisome Proliferator-Activated Receptor (PPAR) [53]. The RET gene encodes a
transmembrane Receptor Tyrosine Kinase (RTK) that is involved in
numerous cellular mechanisms. Its extracellular domain features
four repeats of approximately 110 amino acids, which bear similarities to cadherins. The loss of these genes promotes genetic
instability and is an early event in the carcinogenesis of FVPTC.
There are multiple clinicopathological risk factors associated
with the recurrence of thyroid cancer. Among them are particular
histologic variations, such as the tall cell variant, substantial tumor size, the presence of lymph node metastasis, extrathyroidal
extension, and distant metastasis; all of which are tumor-related
factors [54,55]. Traditional staging methods are not adequate for
assessing recurrence, and recurrent thyroid cancer requires additional therapy and more effective clinical management strategies.
This will have a significant impact on the quality of life of patients
[56]. Understanding somatic mutations in patients with FVPTC
may aid in prognostic risk stratification. Some hospitals recommend the use of targeted next-generation sequencing techniques
to identify thyroid cancer in postoperative tissues as part of determining a patient’s prognosis. Our systematic review indicates
that for FVPTC patients who exclusively harbor RAS mutations
without BRAF or other mutations which are related to malignant
prognosis, ultrasound monitoring and regular follow-up can be
adopted to avoid irreparable damage from overtreatment, such
as direct surgical removal of thyroid tissue. This approach is beneficial in terms of preserving medical resources and reducing the
medical burden on patients. Although we employed various subgroup analysis methods, including tumor preservation conditions,
ethnicity, gene test methods, centers, and quality score, to address the heterogeneity of the meta-analysis concerning frequency, challenges still remained. In contrast, clinical feature-related
meta-analysis showed no heterogeneity across studies, except for
lymph node metastases in BRAF mutation. This finding is a significant result of our study since it underscores the need for a more
detailed understanding of the specific roles of different mutations
in the disease for effective medical treatment. It also highlights
the importance of prioritizing BRAF mutation testing over RAS
mutation testing in FVPTC patients.
Our meta-analysis has some limitations. The research lacked
comprehensive clinical information on treatment techniques and
outcomes for patients. We used eligibility criteria to identify baseline patient features, but we could not determine whether they
underwent surgical or medicinal treatment. Additionally, despite
various subgroup analyses to address this limitation, the heterogeneity of studies regarding mutation frequency is a noteworthy
aspect of our meta-analysis. In contrast, we observed no heterogeneity across studies in relation to clinical features, except for
LNM in BRAF mutation. This finding is significant since patients
with BRAF mutations are found to be at a higher risk of experiencing poor clinical outcomes, unlike RAS mutations which do not
show such an association. Another disadvantage of the study is that some of the research used older techniques for monitoring
gene alterations, such as Sanger sequencing and pyrosequencing,
which only identify 5% to 25% of mutant alleles.
In summary, this study established a somatic mutational landscape for FVPTC. The evidence suggests that FVPTC patients with
BRAF mutations but not RAS mutations have an elevated likelihood of poor clinical characteristics. With its huge sample size,
this study can be used as a reference and guidance for the development of therapeutically targeted treatment medications,
as well as for inclusion in corresponding sequencing panels that
physicians and healthcare regulatory bodies may use.
Declarations
Acknowledgments: We express our gratitude to the participants and those involved in building the resource.
Financial support: This work was supported by the Guangxi
Key Research and Development Program (2021AB07015); Science
and Technology Plan Projects of Liuzhou (2022CAC0229)
Author contributions: Conceptualization, Xiaobo Yang; Formal
analysis, Qinghua Fan and Yi Zhang; Funding acquisition, Xiaobo
Yang and Xiangzhi Li; Methodology, Lulu Huang and Yuwei Jiang;
Software, Fei Wang and Lulu Huang; Visualization, Yuan Zheng,
Huijiao Zhou and Haoyu Wang; Writing – original draft, Qinghua
Fan and Xiuming Feng; Writing – review & editing, Fei Wang. Critical revision and final approval: all authors.
Data availability: All data used in this study are publicly available summary-level data, with the relevant studies cited. Data
that support the findings of our study are available on request
from the corresponding author.
Disclosures: All authors declare to have no conflict of interest.
References
- Lim H, SS Devesa, JA Sosa, D Check, CM Kitahara. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. Jama. 2017; 317(13): 1338-1348.10.1001/jama.2017.2719
- Pontius LN, TO Oyekunle, SM Thomas, MT Stang, RP Scheri, et al. Projecting Survival in Papillary Thyroid Cancer: A Comparison of the Seventh and Eighth Editions of the American Joint Commission on Cancer/Union for International Cancer Control Staging Systems in Two Contemporary National Patient Cohorts. Thyroid. 2017; 27(11): 1408-1416.10.1089/thy.2017.0306
- Miller KD, M Fidler-Benaoudia, TH Keegan, HS Hipp, A Jemal, et al. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020; 70(6): 443-459.10.3322/caac.21637
- Giordano TJ. Genomic Hallmarks of Thyroid Neoplasia. Annu Rev Pathol. 2018; 13: 141-162.10.1146/annurevpathol-121808-102139
- Lam AK, CY Lo, KS Lam. Papillary carcinoma of thyroid: A 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005; 16(4): 323-30.10.1385/ep:16:4:323
- Tielens ET, SI Sherman, RH Hruban, PW Ladenson. Follicular variant of papillary thyroid carcinoma. A clinicopathologic study. Cancer. 1994; 73(2): 424-31.10.1002/1097-0142(19940115)73:2<424::aidcncr2820730230>3.0.co;2-i.
- Chan J. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002; 117(1): 16-8.10.1309/p7ql-16kq-qlf4-xw0m
- Gupta S, O Ajise, L Dultz, B Wang, D Nonaka, et al. Follicular variant of papillary thyroid cancer: Encapsulated, nonencapsulated, and diffuse: distinct biologic and clinical entities. Arch Otolaryngol Head Neck Surg. 2012; 138(3): 227-33.10.1001/archoto.2011.1466
- Vivero M, S Kraft, JA Barletta. Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid. 2013; 23(3): 273-9.10.1089/thy.2012.0369
- Rivera M, J Ricarte-Filho, J Knauf, A Shaha, M Tuttle, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010; 23(9): 1191-200.10.1038/modpathol.2010.112
- Nikiforov YE, RR Seethala, G Tallini, ZW Baloch, F Basolo, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016; 2(8): 1023-9.10.1001/jamaoncol.2016.0386
- Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol. 2016; 29(7): 698-707.10.1038/modpathol.2016.65
- Sicklick JK, S Kato, R Okamura, M Schwaederle, ME Hahn, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019; 25(5): 744-750.10.1038/s41591-019-0407-5
- Pita JM, A Banito, BM Cavaco, V Leite. Gene expression profiling associated with the progression to poorly differentiated thyroid carcinomas. Br J Cancer. 2009; 101(10): 1782-91.10.1038/sj.bjc.6605340
- Park SJ, JY Sun, K Hong, JY Kwak, EK Kim, et al. Application of BRAF, NRAS, KRAS mutations as markers for the detection of papillary thyroid cancer from FNAB specimens by pyrosequencing analysis. Clin Chem Lab Med. 2013; 51(8): 1673-80.10.1515/cclm-2012-0375
- Santarpia L, JN Myers, SI Sherman, F Trimarchi, GL Clayman, et al. Genetic alterations in the RAS/RAF/mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways in the follicular variant of papillary thyroid carcinoma. Cancer. 2010; 116(12): 2974-83.10.1002/cncr.25061
- Bim LV, TNR Carneiro, VC Buzatto, GA Colozza-Gama, FC Koyama, et al. Molecular Signature Expands the Landscape of Driver Negative Thyroid Cancers. Cancers (Basel). 2021; 13(20).10.3390/cancers13205184
- Chang F, LS Steelman, JG Shelton, JT Lee, PM Navolanic, et al. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int J Oncol. 2003; 22(3): 469-80
- Zhang W, HT Liu. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002; 12(1): 9-18.10.1038/sj.cr.7290105
- Indolfi C, EV Avvedimento, A Rapacciuolo, E Di Lorenzo, G Esposito, et al. Inhibition of cellular ras prevents smooth muscle cell proliferation after vascular injury in vivo. Nat Med. 1995; 1(6): 541-5.10.1038/nm0695-541
- McWilliam RA, DJ Baird. Postexposure feeding depression: A new toxicity endpoint for use in laboratory studies with Daphnia magna. Environ Toxicol Chem. 2002; 21(6): 1198-205
- Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010; 321(1): 86-93.10.1016/j.mce.2009.10.012
- Liu X, J Bishop, Y Shan, S Pai, D Liu, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20(4): 603-10.10.1530/erc-13-0210
- Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159(3): 676-90.10.1016/j.cell.2014.09.050
- Kim MJ, JK Won, KC Jung, JH Kim, SW Cho, et al, Clinical Characteristics of Subtypes of Follicular Variant Papillary Thyroid Carcinoma. Thyroid. 2018; 28(3): 311-318.10.1089/thy.2016.0671
- Song YS, JK Won, SK Yoo, KC Jung, MJ Kim, et al. Comprehensive Transcriptomic and Genomic Profiling of Subtypes of Follicular Variant of Papillary Thyroid Carcinoma. Thyroid. 2018; 28(11): 1468-1478.10.1089/thy.2018.0198
- Buryk MA, JP Simons, J Picarsic, SE Monaco, JA Ozolek, et al. Can malignant thyroid nodules be distinguished from benign thyroid nodules in children and adolescents by clinical characteristics? A review of 89 pediatric patients with thyroid nodules. Thyroid. 2015. 25(4): p. 392-400.10.1089/thy.2014.0312
- McFadden DG, D Dias-Santagata, PM Sadow, KD Lynch, C Lubitz, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014; 99(11): E2457-62.10.1210/jc.2014-2611
- Kim TH, M Lee, AY Kwon, JH Choe, JH Kim, et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 2018; 72(4): 648-661.10.1111/his.13401
- Proietti A, R Giannini, C Ugolini, M Miccoli, G Fontanini, et al. BRAF status of follicular variant of papillary thyroid carcinoma and its relationship to its clinical and cytological features. Thyroid. 2010; 20(11): 1263-70.10.1089/thy.2009.0283
- Hahn SY, JH Shin, YL Oh, TH Kim, Y Lim, et al. Role of Ultrasound in Predicting Tumor Invasiveness in Follicular Variant of Papillary Thyroid Carcinoma. Thyroid. 2017; 27(9): 1177-1184.10.1089/thy.2016.0677
- Zou M, EY Baitei, AS Alzahrani, FS BinHumaid, D Alkhafaji, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014; 24(8): 1256-66.10.1089/thy.2013.0610
- Yip L, MN Nikiforova, JY Yoo, KL McCoy, MT Stang, et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015; 262(3): 524-5.10.1097/sla.0000000000001420
- Póvoa AA, E Teixeira, MR Bella-Cueto, R Batista, A Pestana, et al. Genetic Determinants for Prediction of Outcome of Patients with Papillary Thyroid Carcinoma. Cancers (Basel). 2021; 13(9). 10.3390/cancers13092048
- Guan H, G Toraldo, S Cerda, FA Godley, SR Rao, et al. Utilities of RAS Mutations in Preoperative Fine Needle Biopsies for Decision Making for Thyroid Nodule Management: Results from a Single-Center Prospective Cohort. Thyroid. 2020; 30(4): 536-547.10.1089/thy.2019.01164.
- Pokorný J, J Davídek, V Chocholatá, J Pánek, H Bulantová, et al. Interactions of oxidized lipids with protein. Part XVI. Interactions of oxidized ethyl linoleate with collagen. Nahrung. 1990; 34(2): 159-69.10.1002/food.19900340217
- Garcia-Rostan G, H Zhao, RL Camp, M Pollan, A Herrero, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003; 21(17): 3226-35.10.1200/jco.2003.10.130
- Pelizzo MR, IM Boschin, S Barollo, G Pennelli, A Toniato, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor. A mono-institutional experience. Clin Chem Lab Med. 2011; 49(2): 325-9.10.1515/cclm.2011.031
- Qasem E, AK Murugan, H Al-Hindi, M Xing, M Almohanna, et al. TERT promoter mutations in thyroid cancer: a report from a Middle Eastern population. Endocr Relat Cancer. 2015; 22(6): 901-8.10.1530/erc-15-0396
- Proietti A, C Sartori, E Macerola, N Borrelli, G Materazzi, et al. Low frequency of TERT promoter mutations in a series of well-differentiated follicular-patterned thyroid neoplasms. Virchows Arch. 2017; 471(6): 769-773.10.1007/s00428-017-2236-6
- Onder S, O Mete, I Yilmaz, A Bayram, S Bagbudar, et al. DICER1 Mutations Occur in More Than One-Third of Follicular-Patterned Pediatric Papillary Thyroid Carcinomas and Correlate with a Low-Risk Disease and Female Gender Predilection. Endocr Pathol. 2022; 33(4): 437-445.10.1007/s12022-022-09736-y
- Stewart LA, M Clarke, M Rovers, RD Riley, M Simmonds, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. Jama. 2015; 313(16): 1657-65.10.1001/jama.2015.3656
- Fan Qz Yi, Huang Lulu, Feng Xiuming, Zheng Yuan, Zhou Huijiao, et al, Somatic Mutations and Clinical Features of Follicular Variant Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Dataset: figshare. 2023. https://doi.org/10.6084/m9.figshare.22791065.v3
- Sohani ZN, D Meyre, RJ de Souza, PG Joseph, M Gandhi, BB Dennis, et al, Assessing the quality of published genetic association studies in meta-analyses: The quality of genetic studies (Q-Genie) tool. BMC Genet. 2015; 16: 50.10.1186/s12863-015-0211-2
- Mayakonda A, DC Lin, Y Assenov, C Plass, HP Koeffler. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018; 28(11): 1747-1756.10.1101/gr.239244.118
- Finnerty BM, DA Kleiman, T Scognamiglio, A Aronova, T Beninato, et al, Navigating the management of follicular variant papillary thyroid carcinoma subtypes: A classic PTC comparison. Ann Surg Oncol. 2015; 22(4): 1200-6.10.1245/s10434-014-4126-3
- Millington GW. Mutations of the BRAF gene in human cancer, by Davies et al. Nature 2002; 417: 949-54. Clin Exp Dermatol. 2013; 38(2): 222-3.10.1111/ced.12015
- Edlund U, H Grahn. Multivariate data analysis of NMR data. J Pharm Biomed Anal. 1991; 9(8): 655-8.10.1016/0731-7085(91)80191-b
- Mortin LI, PS Stein. Cutaneous dermatomes for initiation of three forms of the scratch reflex in the spinal turtle. J Comp Neurol. 1990; 295(4): 515-29.10.1002/cne.902950402
- Mocellin S, KA Pooley, D Nitti. Telomerase and the search for the end of cancer. Trends Mol Med. 2013; 19(2): 125-33.10.1016/j.molmed.2012.11.006
- Smekalova EM, OS Shubernetskaya, MI Zvereva, EV Gromenko, MP Rubtsova, et al. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc). 2012; 77(10): 1120-8.10.1134/s0006297912100045
- Landa, I, I. Ganly, T.A. Chan, N. Mitsutake, M. Matsuse, T. Ibrahimpasic, et al, Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013; 98(9): p. E1562-6.10.1210/jc.2013-2383
- Kroll TG, P Sarraf, L Pecciarini, CJ Chen, E Mueller, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science. 2000; 289(5483): 1357-60.10.1126/science.289.5483.1357
- Mazzaferri EL, SM Jhiang. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97(5): 418-28.10.1016/0002-9343(94)90321-2
- Zaydfudim VID, Feurer M, R Griffin, JE Phay. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008; 144(6): 1070-1078.10.1016/j.surg.2008.08.034
- Sipos JA, EL Mazzaferri. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol). 2010; 22(6): 395-404.10.1016/j.clon.2010.05.00