Introduction
Overweight and obesity are globally increasingly prevalent
and should be viewed as chronic relapsing diseases that require
continuous efforts toward prevention and treatment [1]. Weight
loss of over 5% is associated with a lower risk of morbidity and
mortality and is known to meaningfully improve health-related
quality of life [2-6]. Lifestyle intervention alone - without any
weight loss surgery, other treatment, or weight loss medication -
results on average in weight loss of 2-5 percent [7] and a gradual
regain of the weight often is common [8]. For those patients who
want or need over 5% sustained weight loss - but are not willing
or eligible to undergo bariatric surgery - weight loss medication
might be an option. One of the popular options is the use of liraglutide (Saxenda). Liraglutide is an acylated Glucagon-Like Peptide-1 (GLP-1) analog that has 97% similarity to human GLP-1 and
is known to lower body weight by reducing appetite and calorie
intake [9]. Randomized controlled trials have proven that liraglutide prescribed up to 3.0 mg a day successfully induced clinically
meaningful weight loss compared to a placebo [10]. The use of
Liraglutide can come with, mostly mild, side effects such as gastrointestinal complaints and headache. These complaints mostly
occur at the start of the treatment but diminish over within weeks
[11]. Aim of the current study is to describe real-world experience
with the use of liraglutide combined with lifestyle coaching in a
bariatric center. In addition, to gain insight in reasons for patients
to when and why they stop using liraglutide.
Materials and methods
Study design
This is a retrospective study including all patients (n=124) who
enrolled in our lifestyle coaching program combined with the use
of liraglutide between July 2019 and July 2021 in our bariatric center. All data were consecutively collected. Informed consent was
given by the patients for anonymous use of their data for research
purposes before the start of the treatment. The study design and
protocol were reviewed and approved by the local Medical Ethics
Committee.
Participants
Participants were patients form a large bariatric center with
locations spread throughout The Netherlands. They were included in the study if they fulfilled all of the following criteria: had a
prescription for liraglutide at the discretion of the physician and
were enrolled in the treatment program between July 2019 and
July 2021, were ≥18 years of age, and, prior to index date, had
BMI≥30 kg/m2
or BMI≥27 kg/m2
with at least one weight-related
comorbidity (e.g., hypertension, T2D, dyslipidemia, sleep apnea).
The complete treatment, medication as well as lifestyle program,
was non-reimbursed for all participants (self-paid). There were no
starting criteria considering previous weight loss attempts or reasons for weight gain (e.g. endocrinological, genetic).
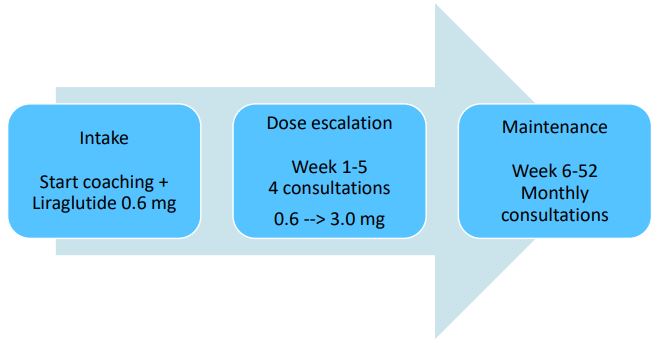
Treatment program
The lifestyle program started with an intake visit with the physician and lifestyle coach. All factors contributing to overweight
(e.g., diet, exercise, mental, social) were explored by using a mind
map and participants were encouraged to identify the factors
they believed they needed to work on. The program consisted of
alternating consultations with the lifestyle coach and the physician. During the first weeks of enrolling in the program and dosage escalation, more frequent consultations were scheduled
(i.e. four sessions during the first 5 weeks) as compared to the
maintenance phase (monthly sessions during the rest of the first
year; Figure 1). Liraglutide treatment initiated at the start of the
coaching program at a daily dose of 0.6 mg, followed by a weekly
dosage escalation of 0.6 mg up to 3.0 mg max or the maximum
tolerable dosage.
Variables
Baseline demographics were collected during the intake visit.
In addition to weight data, the prevalence of symptoms, appetite, satiety, and dosage of liraglutide were structurally noted during
all visits.
Statistical analysis
Baseline demographics and clinical data were reported for all
participants as n (percentage) and mean (SD) or median (interquartile range), as appropriate. A multiple linear regression analysis was performed to determine the predictive value of age, sex
and 12 weeks %TWL on 12 month %TWL. Statistical analysis was
performed using IBM SPSS Statistics for Windows, version 26. The
STROBE cohort reporting guidelines were used [12].
Results
Cohort
Out of the 124 evaluated participants, 82(66%) completed the
12-month lifestyle program while using liraglutide. A total of 31
(25%) participants discontinued the use of liraglutide before the
end of the 12-month program. 11(8.9%) participants were lost
to follow-up, they did not respond to calls and e-mails. On average, participants were 50 years old at inclusion, the majority
was female (82%), and the average starting weight was 105 kg
with a mean starting BMI of 36 kg/m2
. Among all subjects, 15%
were currently being treated for hypertension, 2% for diabetes,
2% had obstructive sleep apnea syndrome, and 8% reported current smoking (Table 1).
Weight loss
Treatment with liraglutide, in addition to lifestyle coaching,
was associated with a median 11.5% TWL after 1 year of treatment. Median decrease in body weight at 4-, 6- and 12-months
post initiation were 8.7%, 9.9% and 11.2%, respectively (Figure 2)
At 6 months after the start of the treatment, 89% had lost at least
5% of their body weight and 52% of participants lost at least 10%.
At 12 months after the start of the treatment this was still 87.8%
with 5% weight loss and 64.6% of participants lost 10%. (Table
2A). Furthermore, a multiple linear regression was run to predict
%TWL at 12 months from age, sex and 12 week %TWL. These variables statistically significantly predicted %TWL at 12 months (R2
=
0.62, F(3,77)=40.99, p<0.000). All three variables added statistically significantly to the prediction, p<0.05. Patients predicted %TWL
at 12 months is equal to -4.169+0.110 (age) – 1.753(Sex) + 1.069
(%TWL at 12 weeks), where Sex is coded as male=0 female=1.
Weight recurrence
After 12 months 51 patients (63%) had gained some weight
compared to the 12 week measurement. No change in weight between these timepoints was seen in 3 patients (3.7%).
Patient experience
Tolerability: The use of liraglutide was well tolerated, with only
mild symptoms, predominantly prevalent during the dosage escalation phase and consisting mainly of gastro-intestinal symptoms
and fatigue (Table 2B).
Effect: Participants reported to feel less hungry (61.3%) or to
have no appetite at all (35.6%). In addition, satiety was reported
to be better in 95.2% of the participants (Table 2B). Unfortunately,
monitoring comorbidities was not part of the follow-up.
Dosage: Within the first 4 months of their treatment, a total of
9(7.3%) participants discontinued the use of liraglutide. Of the remaining 115 participants who were using liraglutide at 4 months
post-initiation, 92(80%) reached the dose of 3.0 mg daily, whereas
7(6%) were still using 0.6 mg, 8(7%) were using 1.8 mg and another 8 (7%) were using 2.4 mg. Reasons for not uptitrating to
the maximum dosage were mainly: already experiencing enough
satiety and weight loss at a lower dose, costs, or because of symptoms (Table 2B).
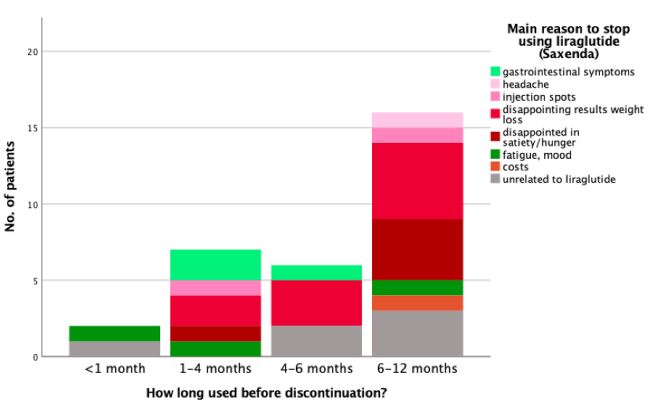
Discontinuation of liraglutide: A total of 31(25%) participants
stopped using liraglutide during the 12-month program. The majority of those who stopped, discontinued between 6-12 months
post-initiation (51.6%) (Table 2B). Reasons to stop using liraglutide were mainly being disappointed with the weight loss result
(32.2%) or with the effect on appetite/satiety (16.1%). Furthermore, a total of 6 patients chose to discontinue their liraglutide
use for reasons unrelated to the treatment, such as divorce, malignity, death of spouse, and COVID (Table 2B). Reasons for discontinuing liraglutide are not clearly associated with the amount
of time that it had been used before discontinuation (Figure 3).
Table 1: Baseline characteristics.
|
Baseline characteristics
|
n=124 |
| Age, yrs, mean (SD) |
50.1(11.1) |
| Gender, female (%) |
101(82) |
| Weight, kg, mean (SD) |
104.9(20.4) |
| BMI, kg/m2, mean (SD) |
36.1(5.6) |
| Hypertension (%) |
19(15.3) |
| Diabetes (%) |
2(1.6) |
| Dyslipidemia (%) |
11(8.9) |
|
Obstructive sleep apnea
syndrome (%)
|
3(2.4) |
| Smoking (%) |
10(8.1) |
SD: Standard Deviation
Table 2A: Baseline characteristics.
| Weight variables |
Median [min-max] (IQR) |
n |
| Start weight (kg) |
102.5 [73.0-180.0]
(88.7-114.1)
|
124 |
| Start BMI |
34.9 [27.3-60.3] (32.4-38.6)
|
124 |
|
Weight loss after 4 months
(kg)
|
9.1 [1.0-27.1] (6.1-12.0)
|
113(91%) |
| TWL after 4 months (%) |
8.7 [1.0-22.4] (6.3-11.4)
|
113(91%) |
|
Weight loss after 6 months
(kg)
|
10.7 [2.0-33.1] (7.5-13.8)
|
102(82%) |
| TWL after 6 months (%) |
9.9 [1.9-27.4] (7.1-14.1)
|
102(82%) |
|
Weight loss after 12 months
(kg)
|
12.0 [-7.0-37.0] (9.0-15.0)
|
82(66%) |
| TWL after 12 months (%) |
11.5 [-6.0-30.1] (8.5-15.6)
|
82(66%) |
|
At least 5% TWL after 4
months, n (%)
|
100(80.6) |
113(91%) |
|
At least 5% TWL after 6
months, n (%)
|
91(89.2) |
102(82%) |
|
At least 5% TWL after 12
months, n (%)
|
72(87.8) |
82(66.1%) |
|
At least 10% TWL after 6
months, n (%)
|
52(51.0) |
102(82%) |
|
At least 10% TWL after 12
months, n (%)
|
53(64.6%) |
82(66.1%) |
TWL: Total weight loss (%).
Table 2B: Treatment.
| Patient reports |
|
|
Tolerability: symptoms
during 1st month
|
n=124 |
| Nausea, n (%) |
84(68.3) |
| Vomiting, n (%) |
4(3.3) |
| Pyrosis, n (%) |
18(14.6) |
| Fatigue, n (%) |
20(16.3) |
| Constipation, n (%) |
41(33.3) |
| Dizziness, n (%) |
11(8.9) |
|
Effect: perception of
appetite*
|
n=124 |
| No appetite, n (%) |
43(34.7) |
| Less hungry, n (%) |
76(61.3) |
|
Same appetite as before
start treatment, n (%)
|
5(4.1) |
|
Effect: perception of
satiety*
|
n=124 |
|
No effect on satiety, n (%)
|
6(4.8) |
| More satisfied, n (%) |
118(95.2) |
|
Dosage at 4 months, n (%)
|
n=115 |
| 0.6 mg |
7(6.1) |
| 1.2 mg |
0 |
| 1.8 mg |
8(7.0) |
| 2.4 mg |
8(7.0) |
| 3.0 mg |
92(80.0) |
|
Liraglutide discontinued, n
(%)
|
31(25) |
|
Stopped during 1st month, n
(%)
|
2(1.6) |
|
Stopped between 1-4 months
of use, n (%)
|
7(5.6) |
|
Stopped between 4-6 months
of use, n (%)
|
6(4.8) |
|
Stopped between 6-12 months
of use, n (%)
|
16(12.9) |
|
Reasons for discontinuation
|
|
|
Gastrointestinal symptoms, n
(%)
|
3(9.7) |
| Headache, n (%) |
1(3.2) |
| Injection spots, n (%) |
2(6.5) |
|
Disappointing results weight
loss, n (%)
|
10(32.3) |
|
Disappointed effect
appetite/satiety, n (%)
|
5(16.1) |
| Fatigue, mood, n (%) |
3(9.7) |
| Costs, n (%) |
1(3.2) |
|
Unrelated to Liraglutide, n
(%)
|
6(19.4) |
*Patient reported, during uptritation phase.
Discussion
Liraglutide with lifestyle coaching is effective, well-tolerated,
and associated with clinically meaningful weight loss in people
who are overweight or living with obesity. The majority (81%) of
patients reach at least 5% TWL at 4 months post-initiation. After
6 months of treatment, more than half of them reached at least
10% TWL (51%). Reasons for patients to stop treatment with liraglutide are disappointment in weight loss or effect on satiety and
appetite, mainly after the first 6 months of use.
The current weight loss result is in line with earlier publications
in various clinical and controlled settings [10,13]. At our center,
we believe that specifically the combination of weight loss medication with lifestyle coaching is crucial for achieving solid weight
loss and maintaining it. This statement is supported by a recent
study, in which participants were prescribed liraglutide 3.0 mg for
weight loss. Their weight measurements were taken for a period
of 6 months after initiation. In this study, no structured lifestyle
coaching was done- instead, patients were instructed ‘to maintain
a healthy lifestyle, such as avoiding high-calorie and high-fat diet,
and by undertaking regular exercise’ [14]. Although weight loss
after 6 months was still significant- average TWL of 6% while 53%
achieved at least 5% body weight loss- the results would probably have been better with more coaching. Moreover, a recent
paper by Capristo et. al underlined the effect of lifestyle coaching
in addition to the use of liraglutide by demonstrating that very
comprehensive lifestyle modifications- i.e., prescription of a very
low-calorie diet and intensive sports regime combined with liraglutide use - resulted in an average of 24% TWL [15].
Frequent coaching is especially crucial at the start of liraglutide
treatment. First, this is the phase of uptitration and therefore this
is the moment that patients need more motivation and reassurance regarding potential side effects. Second, it is well known that
early weight loss is considered to be predictive of a better longterm weight loss [16,17].
In our cohort, 80% reached the dose of 3.0 mg daily. Reasons
for not uptitrating to the maximum dosage were patient driven.
This stresses the importance of adequate guidance of patients and
an individual approach for optimal, personalized care. Already in
the 70s of the previous century, it was demonstrated that when
verbal reinforcement and positive feedback were used, intrinsic
motivation tended to increase [18].
Over the course of one year, 25% discontinued the use of liraglutide for various reasons and at different time points. This
proportion is in line with other publications [14,19]. The majority of participants who chose to stop using liraglutide in the second half of the year were disappointed in the weight loss and/or
the amount of satiety that they experienced. In the study from
Rubino et al. reasons such as personal issues are mentioned and
a patient that wished to undergo surgical treatment instead of
continuing with medication. On the other hand, discontinuation
because of gastrointestinal symptoms occurred only in the first 6
months. This is in line with the randomized clinical trial of Rubino
et al., demonstrating the decrease of gastrointestinal symptoms
after the first weeks of use [19].
Limitations of the study
It is important to note that for all participants, the treatment program as well as the use of liraglutide were not reimbursed, therefore,
study participants may represent a population more motivated to
lose weight than the general eligible population as was confirmed
in one study on the effect of funding on weight loss after gastric
banding: Self-pay patients initially achieved more weight loss [20].
Moreover, our results may not be generalizable to patients who
are living with more severe obesity (BMI>50) or younger patients.
In the current study the comorbidities were not monitored over
time. Therefore, no conclusions can be made on the effect of liraglutide treatment on comorbidity reduction or remission. However, literature describes a positive effect of weight loss on the
reduction of comorbidities in patients with obesity [21,22].
Conclusion
The use of liraglutide is well-tolerated and associated with
clinically meaningful weight loss in a cohort with a mean BMI of
36 kg/m2
. Gastrointestinal symptoms are not the main reason to
stop using liraglutide. Reasons for patients to stop treatment with
liraglutide are disappointment in weight loss or effect on satiety
and appetite, predominantly after 6 months of use.
Declarations
Conflict of interest statements: The authors have no conflicts
of interest to declare.
Funding: No funding.
Acknowledgements: We would like to thank the NOK Clinics
teams for their excellent patient care. The author contribution
was as follows: IHPM was responsible for designing and writing
the protocol, extracting and analyzing data, interpreting results,
updating reference lists and writing the paper. MTFJ contributed
to writing the protocol, interpreting results, and provided feedback on the paper. JWMG and EJGB contributed to designing the
study, data interpretation and provided feedback on the report.
References
- Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017; 376: 254-66.
- Poobalan AS, Aucott LS, Smith WC, et al. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007; 8: 503-13.
- Wright F, Boyle S, Baxter K, et al. Understanding the relationship between weight loss, emotional well-being and health-related quality of life in patients attending a specialist obesity weight management service. J Health Psychol. 2013; 18: 574-86.
- Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: A meta-analysis. Am J Clin Nutr. 1992; 56(2): 320-8
- Wing RR, Lang W, Wadden TA, Safford M, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011; 34(7): 1481-6.
- Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014; 15(3): 169-82.
- Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obes Res. 2001; 9(4): 326S334S.
- Dombrowski SU, Knittle K, Avenell A, et al. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ. 2014; 348: g2646.
- Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014; 38: 784-793.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0mg of liraglutide in weight management. N Engl J Med. 2015; 373(1): 11-22.
- Sm PC Saxenda 6mg/ml solution for injection in pre filled pen. Novo Nordisk Limited. 2022.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies.
- Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015; 314(7): 687-99.
- Jung Ha Park, Ju Young Kim, et al. Effectiveness of liraglutide 3 mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention. International Journal of Obesity. 2021; 45(4): 776-786.
- Capristo E. Intensive lifestyle modifications with or withour liraglutide 3 mg vs. sleeve gastrectomy: A three arm non-randomised, controlled, pilot study. Diabetes and Metabolism. 2018; 44: 235-242.
- Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, et al. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obesity. 2020; 28(3): 529-536.
- Maccora C, Ciuoli C, Goracci A, et al. One month weight loss predicts the efficacy of Liraglutide in obese patients: data from a single center. Endocr Pract. 2020; 26(2): 235-240.
- Deci E. Effects of externally mediated rewards on intrinsic motivation. Journal of Personality and Social Psychology. 1971; 18(1): 105-115.
- Rubino M, Greenway F, Khalid U, et al. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022; 327(2): 138-150.
- Afoke J, Agrawal S, Edmond J, Mahon D, Welbourn R. Effect of source of funding on weight loss up to 3 years after gastric banding. Surg Endosc. 2013; 27(4): 1219-24.
- Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999; 7(5): 477-84. doi: 10.1002/j.1550-8528.
- Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep. 2017; 6(2): 187-194. doi: 10.1007/s13679-017-0262-y.