Introduction
Pancreas transplantation is unique from other solid organ
transplants such as the liver, kidney, heart or lung because the
entire organ is usually not needed for the purpose of transplantation. This 100 gram organ consists of two types of tissues with
different functions: exocrine and endocrine. Ninety-eight percent
of the pancreas volume is exocrine tissue and only one or two percent is endocrine tissue. The vast majority of pancreas transplants
is performed to replace the endocrine function of the pancreas in
diabetic patients. However, for less common indications, a pancreas transplant may be performed to restore both the exocrine
and the endocrine function of the pancreas [1].
Pancreas transplant options include: (1) segmental pancreas
autotransplants or islet autotransplant in patients with chronic
pancreatitis; (2) pancreas allotransplants for selected patients
with a previous native pancreatectomy to treat chronic pancreatitis; (3) pancreas autotransplant in selected patients with malignancy; and (4) pancreas or islet allotransplants (as part of “cluster” transplants) in selected patients to treat upper-abdominal
malignancies.
Chronic pancreatitis
Chronic pancreatitis is a progressive fibro-inflammatory disease that causes the destruction of pancreatic exocrine and endocrine tissue. Eventually, the secretory parenchyma is replaced
with fibrotic tissue. Chronic pancreatitis comprises a number
of etiologies and classifications. According to one classification,
three types can be distinguished: Chronic calcifying pancreatitis,
chronic obstructive pancreatitis, and steroid-responsive pancreatitis (chronic autoimmune).
Genetic, metabolic, environmental, toxic and/or other risk
factors can lead to persistent pathologic changes of the pancreas including severe parenchymal injury [2]. Advanced stages of
chronic pancreatitis may include histopathological changes such
as pancreatic atrophy, fibrosis, duct distortion and strictures, calcifications, pancreatic exocrine dysfunction, pancreatic endocrine
dysfunction, dysplasia; once diagnosed, these changes are irreversible [1]. The most distressing feature of chronic pancreatitis
and recurrent episodes of acute pancreatitis is intractable pain,
resulting in opioid addiction, extremely poor quality of life and
disability, all of which are usually worse than those seen in other
common chronic disorders and cancer [3-5]. The progressive inflammatory acinar process eventually impacts the beta cells as
well and may cause brittle diabetes mellitus [6-8].
Chronic calcifying pancreatitis is the most common type of
chronic pancreatitis. It causes development of stones in the main
pancreatic duct and/or in the side branches and may result in
pancreatic duct distortion, stricture, and pancreatic atrophy. In
contrast, obstructive or autoimmune chronic pancreatitis rarely
cause calcifications of the pancreas.
Chronic obstructive pancreatitis usually results from primary
injury to the duct or from partial or complete ductal obstruction
[9-11]. Obstructive pancreatitis occurs upstream from a pancreatic duct stricture. It is caused by pancreatic duct injury for a variety of reasons including endoscopic or surgical procedures, acute
necrotizing pancreatitis, blunt abdominal trauma, narrowed pancreatico-enteric anastomoses and tumors obstructing the pancreatic duct (eg, ductal adenocarcinoma or intraductal papillary
mucinous tumor). Ductal obstruction due to strictures and stones
can also cause chronic calcifying pancreatitis. In the typical form
of chronic obstructive pancreatitis, only the organ upstream from
the obstruction is affected, with the downstream pancreas being
healthy and of normal appearance.
Steroid-responsive or autoimmune chronic pancreatitis is a
type of chronic pancreatitis that responds well to corticosteroid
therapy. Autoimmune pancreatitis is categorized in two types:
type 1 and type 2. They are different entities. Type 1 is generally
associated with true autoimmune pancreatitis and it has been suggested to call this form autoimmune pancreatitis whereas type 2
should be called idiopathic duct-centric chronic pancreatitis [12].
Type 1 steroid-responsive chronic pancreatitis is the pancreatic manifestation of a multiorgan fibro-inflammatory syndrome
known as immunoglobulin G4-related syndrome. This syndrome
presents with multiorgan involvement, characteristic histology, an
increase in serum IgG4 levels, and a rapid response to corticosteroid. The IgG-4 related disease manifests itself in several organs
such as the pancreas, bile ducts, salivary glands, retroperitoneum,
kidneys, and lymph nodes [13]. The histopathology shows dense
lymphoplasmacytic infiltrates around the mid-size ducts, a peculiar swirling (storiform) fibrosis, an intense inflammation that
surrounds the veins and spares adjacent arteries, and frequent IgG4 plasma cells. The most common symptom of type 1 autoimmune pancreatitis is obstructive jaundice. Less often, it presents
with acute pancreatitis. Pain is not severe nor as common as with
other types of chronic pancreatitis and resolves rapidly with corticosteroid therapy. Calcification is not frequently observed and
may occur after multiple relapses of the disease [14].
Idiopathic duct-centric chronic pancreatitis (type 2) differs substantially from type 1 autoimmune chronic pancreatitis. Histopathology of this type shows a picture in which the pancreatic duct
epithelium is infiltrated by neutrophils. Type 2 chronic pancreatitis inclines to cause multiple recurrent bouts of acute pancreatitis.
According to the Pancreas Foundation pancreasfoundation.org [15], the annual incidence rate of chronic pancreatitis
is 5-12/100,000 people in industrialized nations. The prevalence
of chronic pancreatitis is 50/100,000 people. Chronic pancreatitis
often develops in patients between the ages of 30 and 40, and is
more common in men than women.
Factors that increase the risk of chronic pancreatitis are alcohol, smoking, autoimmune and anatomical abnormalities, but
genetic factors were also well recognized. Genetic variations associated with chronic pancreatitis are PRSS1 (Protease, Serine 1,
a cationic trypsinogen), SPINK 1 (serine protease inhibitor kazaltype 1), and CFTR (cystic fibrosis transmembrane conductance
regulator) and, to a lesser degree, CTRC (chymotrypsin C) and
CASR (calcium-sensing receptor) [16]. The polymorphism and
mutation have several mechanisms and variations. The most recognized gene associated with chronic pancreatitis is PRSS 1. More
than 40 mutations in the PRSS1 gene have been found to cause
hereditary pancreatitis and most of these mutations change single protein building blocks (amino acids in cationic trypsinogen).
Some PRSS1 gene mutations result in the production of a cationic trypsinogen enzyme that is prematurely converted to trypsin
while it is still in the pancreas. Other mutations prevent trypsin
from being broken down. The most common PRSS1 gene mutation that causes hereditary pancreatitis replaces the amino acid
arginine with the amino acid histidine at position 122 in the enzyme (written Arg122His or R122H). As a result of this mutation,
the enzyme cannot be broken down, even when it is no longer
bound to calcium. Genetic chronic pancreatitis has a different
course than other forms of chronic pancreatitis. It is associated
with early onset, rapid progression to chronic pancreatitis and a
high risk of pancreatic adenocarcinoma. Genetic testing may be
considered in patients with pancreatitis at age below 25 who have
had recurrent episodes of acute pancreatitis with an idiopathic
etiology [17].
Medical therapy for chronic pancreatitis
Lifestyle modifications, cessation of alcohol use and smoking,
exercise, avoiding weight gain and a multidisciplinary approach by
a dedicated chronic pancreatitis team including surgeon, gastroenterologist, dietician, social worker, psychologist, pain management specialist and pharmacist are essential in the management
of this complicated chronic disease.
The medical treatment of chronic pancreatitis can be categorized according to treatment of (1) pain, (2) exocrine and endocrine deficiency, and (3) complications of chronic pancreatitis
such as bleeding, obstruction and cancer.
Severe abdominal pain is the major complaint and it is experienced in 50-85% of patients. Management of abdominal pain is
challenging and has a high rate of failure [18]. Ceyhan et al. [19]
showed that pancreatic sympathetic innervation was significantly
reduced in chronic pancreatitis and pancreatic cancer, whereas
parasympathetic innervation did not show major changes. Nestin
neuro-immunoreactivity was stronger, and Sox10-immunoreactivity was weaker in chronic pancreatitis and pancreatic cancer
than in normal pancreata. Pancreatic sympathetic and cholinergic innervation was noticeably decreased in patients with severe
pancreatic neuritis, neural invasion by cancer cells, or abdominal
pain. Moreover, the neural immunoreactivity for Nestin and Sox10
also varied with intrapancreatic neuropathic alterations and abdominal pain. Other studies showed that in chronic pancreatitis,
intrapancreatic nerves are remarkably enlarged and increased
in number and the structure of the intra-pancreatic nerves was
altered [20,21]. Nerves are frequently surrounded by inflammatory cells that often infiltrate these nerves through a damaged
perineurium and cause the characteristic pancreatic neuritis [22].
The important role of these neural and perineural alterations
in the pathogenesis of pain in chronic pancreatitis was already
suggested in the mid-1980s. More recent studies have shown a
positive correlation between these neuromorphological changes
in chronic pancreatitis and the degree of pain experienced by patients [23,24].
Several treatment options have been suggested to manage
abdominal pain in patients with chronic pancreatitis. Opioids
can lead to tolerance and dependence and should be carefully
assessed before utilization. Tricyclic antidepressants, selective
serotonin-reuptake inhibitors, gabapentin, and pregabalin have
been used either alone or in combination with opioids with different outcomes. Winstead and Wilcox [25] reviewed the literature regarding the use of pancreatic enzymes in the treatment
of chronic pancreatitis pain. They recommended that the pain
should be assessed in a standardized and repeatable fashion prior
to initiating a therapeutic trial of pancreatic enzymes. Therapeutic
trials should be limited to 6 weeks with uncoated enzymes and
concurrent acid suppression, at which point another standardized
pain measurement questionnaire should be completed. They also
suggested that one group of patients is not more likely to benefit
from this intervention than another; however, it may be more effective for women with nonalcoholic chronic pancreatitis. Since
only this one report regarding uncoated enzyme therapy showed
significant improvement in pancreatic pain management, they did
not recommend routine use of pancreatic enzymes in the treatment of painful chronic pancreatitis.
A double-blinded, randomized, controlled trial, the ANTICIPATE
study [26], reported in 70 patients at 6 months a reduction of pain
scores by 1.97 from baseline in the placebo group and by 2.33 in
the antioxidant group, but there was no statistically significant difference between the groups (-0.36; 95% Confidence Interval [CI],
-1.44 to 0.72; P=.509). The average daily pain scores from diaries
were also similar (3.05 for the placebo group and 2.93 for the antioxidant group, a difference of p=0.11; 95% CI, 1.05-0.82; P=.808).
Measures of quality of life were similar between groups, as was
opiate use and number of hospital admissions and outpatient
visits. Blood levels of vitamin C and E, β-carotene, and selenium
were increased significantly in the antioxidant group. However,
the use of antioxidants did not reduce the pain or improve quality of life, despite increase of the antioxidant in the blood.
Thoracoscopic splanchnicectomy was first described as minimally invasive therapy for pain in chronic pancreatitis in 1994
[27]. With this procedure, the nociceptive input of the pancreas
is interrupted by denervating the splanchnic nerves at the level of
the thorax before they enter the sympathetic cord. Some studies
showed relief of pain by this procedure for short-term [28,29].
However, the long-term outcome did not reveal a significant reduction of the chronic pancreatic pain. New evidence suggests
that the failure of this procedure results from prolonged use of
opioids that sensitizes the peripheral nerve, leading to permanent
hyperalgesia that is difficult to cure and reverse [30-32].
Surgical interventions for chronic pancreatitis
Non-transplant surgical options
A good number of patients with chronic pancreatitis may not
respond to multiple medical therapies [33]. Surgical interventions
may be useful in selected patients. Different surgical interventions
are recommended for patients with poorly controlled abdominal
pain, duodenal, biliary or pancreatic obstruction, symptomatic
pseudocyst, or suspicion of cancer. The surgical interventions that
are commonly used can be classified into 4 categories: (1) drainage procedures, (2) partial pancreatic resection, (3) a combination
thereof and (4) total pancreatectomy with or without islet autotransplantation.
The techniques for drainage procedures have evolved over
time. Du Val and Zollinger et al. almost simultaneously described
retrograde pancreatic duct drainage into a defunctionalized jejunal loop. This procedure includes resection of both a (small)
portion of the pancreatic tail and spleen [34,35]. Puestow et al.
described caudal pancreaticojejunostomy to drain multiple strictures and dilatations (a so-called “chain of lakes”) frequently associated with chronic pancreatitis: the pancreatic duct is opened
longitudinally from the transected tail to a point just to the right
of the superior mesenteric vessels; this portion of the pancreas is
anastomosed to the end of a jejunal Roux-en-Y loop [36]. Partington and Rochelle described a side-to-side pancreaticojejunostomy
with the pancreatic duct opened all the way from the pancreatic
tail to its entry into the duodenum; resection of any portion of the
pancreatic tail or spleen is not required [37].
Drainage procedures may be combined, not only with resection of a small portion of the tail of the pancreas (as described by
Du Val, Zollinger et al., and Puestow et al.), but also with (partial)
resection of the pancreatic head. Indeed, the head of the pancreas has been coined “the pacemaker” of chronic pancreatitis.
Up to 35% of patients develop an inflammatory mass with an enlarged pancreatic head. The combination of a drainage procedure
and resection of the anterior portion of the pancreatic head was
described by Frey and Smith [38]. Coring out the pancreatic head
and the uncinate process not only removes diseased tissue but
also allows drainage of Wirsung’s duct, Santorini’s duct, the duct
to the uncinate process, and their tributary ducts. The unroofed
pancreatic ducts and the partially resected pancreatic head with
the uncinate process are drained side-to-side using a jejunal
Roux-en-Y loop.
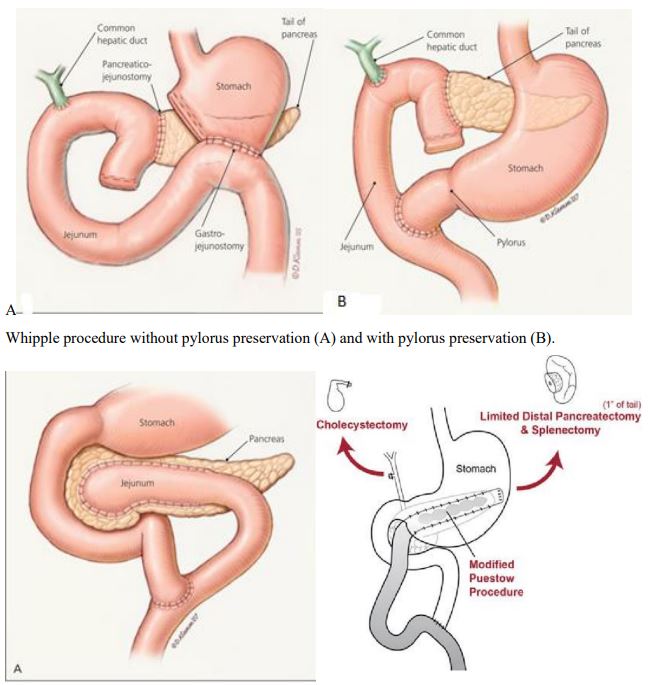
Resective procedures can be classified into two groups: (1) partial resection of the pancreas (e.g., standard Whipple procedure, pylorus- or duodenum-preserving resection of the pancreatic
head, distal pancreatectomy) (Figures 1 and 2) complete resection of the pancreas (with or without preservation of the pylorus
or duodenum). Advantages of partial resection are that (1) almost
half of the pancreatic tissue is left behind and (2) patients may
not develop exocrine or endocrine deficiency. However, the incidence of endocrine insufficiency after partial resection increases
with time.
The standard Whipple procedure [39] or the duodenum-preserving resection of the pancreatic head (to Beger et al. [40] is
performed if chronic pancreatitis is predominantly located in the
pancreatic head (frequently associated with an enlarged, inflammatory mass) and if the tail of the pancreas shows little evidence
of chronic pancreatitis. The advantage of a duodenum-preserving
resection is that it excludes surgery of the stomach, duodenum,
and biliary tree, but it requires creation of two pancreatic anastomoses (one to the distal pancreas and one to the remnant of the
pancreatic head). In contrast, the standard Whipple procedure
(with or without pylorus preservation) removes the whole pancreatic head, involves surgery of the biliary tree, and is associated
with a higher rate of endocrine insufficiency.
Distal pancreatectomy is indicated for patients whose disease
process is mainly confined to the distal pancreas. Its morbidity
and mortality are lower than for resective procedures of the head,
and it only infrequently exacerbates exocrine or endocrine insufficiency. However, radical distal pancreatectomy (85% to 90% resection) carries a much higher risk of exocrine or endocrine insufficiency than standard distal pancreatectomy (40% to 60% resection). Distal pancreatectomy for chronic pancreatitis can be done
with or without spleen preservation. Of note, about 20% to 40%
of patients with partial or complete resection of the pancreatic
head show, on further imaging, progressive changes of chronic pancreatitis in the pancreatic body and tail within 6 to 12 months
after the initial resection. Such patients often experience recurrent pain and may require a completion distal pancreatectomy.
Vice versa, if, after distal pancreatectomy, subsequent imaging of
the pancreatic head shows evidence of progressive pancreatitis
and if severe pain recurs, then a completion proximal pancreatectomy may be indicated. In addition, diabetes is estimated to develop long-term in 80% of patients who undergo near-total (i.e.,
80% to 95%) distal resection.
Total pancreatectomy remains the surgeon’s last resort in the
treatment of chronic pancreatitis. This procedure inevitably results in complete exocrine and endocrine insufficiency of the pancreas. A “radical” therapy for a “benign” disease, it is still associated with a high morbidity and mortality rate. In addition, up to
one third of patients do not achieve pain relief and continue to require opiate-driven analgesia [8-11]. Total pancreatectomy is usually performed only after all other treatment modalities (including
the resective procedures above) have failed. Three techniques for
complete removal of the pancreas have been described: (1) duodenum-preserving total pancreatectomy, which involves dissection of the distal bile duct away from the pancreas and resection
of the pancreatic tissue, by sharp dissection, between the bile
duct and the first and second parts of the duodenum; (2) pyloruspreserving total pancreatectomy in which jejunum is brought up
from the ligament of Treitz and anastomosed end-to-end with the
first portion of the duodenum, and an end-to-side choledochojejunostomy is created 10 cm distally; and (3) total pancreatectomy,
without preservation of the duodenum or pylorus [41-44].
After total pancreatectomy, treatment of exocrine insufficiency
is usually considered easier than treatment of the ensuing surgical diabetes mellitus. Patients frequently develop a brittle form
of diabetes mellitus: they are particularly sensitive to insulin and
prone to hypoglycemic episodes because of the lack of other glucose regulatory hormones such as glucagon. As a consequence,
they show wide oscillations between hypo- and hyperglycemia.
Hospitalizations because of hypoglycemia, ketoacidosis, and failure to thrive are not uncommon. In fact, hypoglycemic unawareness is a well-described cause of death after total pancreatectomy
[45,46].
A good number of patients suffering from chronic pancreatitis
continue to be devastated by the pain and poor quality of life despite much surgical and medical therapy [47-50].
Drainage procedures usually provide good long-term pain relief in only 10% to 30% of patients and partial or complete resection of the pancreas in 20% to 50%.
Total Pancreatectomy with Islet Auto-Transplant (TPIAT)
Pain relief is the primary objective of surgery for chronic pancreatitis. An additional objective of pancreas or islet autotransplant,
alternatives to the classic drainage and resective procedures, is
to prevent severe endocrine deficiency. Patients scheduled to undergo total or near-total pancreatectomy might as well undergo
a simultaneous pancreas or islet auto-transplant, which, in addition, offers the chance of being insulin independent or at least
makes their diabetes easier to manage. But, surprisingly, despite
the success of simultaneous pancreas or islet autotransplant, they
are still not mentioned in many standard textbooks of surgery. Moran et al. studied 46 patients who underwent total pancreatectomy and autoislet transplant. They showed that, following
surgery, 89% of patients had resolution of their pre-operative
abdominal pain; however, 83% of patients developed a different
form of abdominal pain. Opioid independence was achieved in
46% of patients. Acute recurrent pancreatitis (OR: 11.66; 95% CI:
1.47-92.39; p=0.02) but not pain duration >3 years or ≥ 5 ERCPs
was independently associated with resolution of pre-operative
abdominal pain on multiple logistic regression. None of these factors were associated with cessation of opioid use [51].
After total pancreatectomy, patients will develop type 3c brittle diabetes with widely-fluctuating blood sugar levels that are
very difficult to control due to removal of both insulin and glucagon secreting cells [52]. For this reason, islet-auto transplant
is offered to these patients to restore euglycemia [53-55]. However, some patients with chronic pancreatitis already suffer from
diabetes due to inflammatory and fibrosis damage to islet cells
and therefore, islet auto-transplant is not feasible. Furthermore,
in many patients, an optimal islet cell yield cannot be obtained for
auto-transplantation due to the severely damaged endocrine cells
caused by chronic pancreatitis.
Adams et. al. [56] reported that of 160 patients with total
pancreatectomy and islet auto-transplant, 73(48.8%) developed significant side effects including delayed gastric emptying
in 20(12.5%) patients, pneumonia in 23(14.4%), intraabdominal abscesses in 10(6.25%), unplanned reintubation in 9(5.6%),
acute renal failure in 8(5.0%), septic shock in 6(3.8%) and wound
infection in 6(3.8%). Post-operative hospital length of stay was
12.4±1.0 days, reoperation was required in 17 patients (10.6%)
and readmission in 46 patients (28.8%). Thirty-day mortality was
observed in 2 patients (1.25%) and 90-day mortality in 4 patients
(2.5%.) One hundred and sixty patients were available for longterm follow-up, of whom 13 patients died (8.1%). The median duration of follow-up was 4.8±0.2 years. They concluded that total
pancreatectomy with islet auto-transplant has its own significant
side effects.
In another study by Al‐sofiani et al, only one-third of their patients achieved insulin independence and up to 75% required insulin therapy after islet auto- transplant [57].
Total pancreatectomy and islet auto-transplant does not cure
exocrine insufficiency. Thus, the most biological therapy to replace the removed pancreas is pancreas transplantation to restore both endocrine and exocrine function of the pancreas. The
disadvantage of this therapy is surgery and immunosuppression.
Pancreas transplant surgery has evolved over time due to many
advances in surgical techniques [58] and surgical complications
resulting in pancreas graft loss have declined to less than 10% at
the majority of transplant centers. Furthermore, the rate of graft
loss from rejection has also significantly declined to 3% in SPK and
8% in PAK and 15% in PTA recipients [59].
Pancreas allotransplantation after total pancreatectomy for
chronic pancreatitis
Most pancreas transplants are performed to cure diabetes;
only 0.1% of pancreas transplants have been performed after total pancreatectomy in the US (Table 1).
The first pancreas transplant after total pancreatectomy was
reported in 1991 by Dr. Gruessner from the University of Minnesota [60].
In 2008, Gruessner et al. reported a series of 26 patients who
underwent a total pancreatectomy and a subsequent pancreas allotransplant. In his report, patient survival rates at 1- and 3-years
in both the CSA and TAC eras were 100% and 100%; in the CNIfree era, at 1 year, the survival rate was lower due to the small
number of transplants. Pancreas graft survival rates in the CSA era
were 67% and 50% at 1 and 3 years, respectively; in the TAC era,
73% and 51%, respectively; and in the CNI-free era, at 1 year, 40%
(p=0.13). The mean number of rejection episodes in the CSA era
was 2.1; in the TAC era, 1.4; and in the CNI-free era, 0.6. It was
concluded that (1) pancreas allotransplants in patients with a previous total pancreatectomy for chronic pancreatitis can achieve
pancreas graft survival rates of 70% with TAC-based immunosuppression; (2) pancreas transplants can successfully treat both endocrine and exocrine insufficiency; and (3) sequential pancreas allotransplants should be considered a treatment option in patients
with pancreatectomy-induced brittle diabetes mellitus or with
progression of secondary complications of diabetes mellitus [61].
In a European study [62], eight patients (1.4% of total pancreas
transplants) underwent pancreas transplant alone after total pancreatectomy due to chronic pancreatitis. Patient and graft survival
rates were 88% and 88% at 1-year and 88% and 75% at 3-years,
respectively. One patient died due to sepsis caused by vancomycin-resident bacteremia and subsequent graft-versus-host disease almost one year after the transplant. Median hospital stay,
rejection and infection rates were not different than for pancreas
transplants without prior pancreatectomy. Seventy-five percent of
the patients remained insulin-free for up to 5-years. Seventy-five
percent of patients with documented pancreatic enzyme supplement use pre-transplant did not need further pancreatic enzyme
supplementation post-transplant. Thirty-three percent of patients
could be weaned off from narcotic medications for pain control.
Cystic fibrosis
According to the International Pancreas Transplant Registry
(IPTR), 26 transplants including the pancreas were performed for
cystic fibrosis in the U.S. between January 1988 and December 2020 (Table 2). There were 6 transplants in the traditional pancreas transplant categories (SPK 3, PAK 2, PTA 1) and 20 various
multiorgan transplants including the pancreas. Of the 20 pancreas-multiorgan transplants, only 1 (a combined pancreas-intestine
transplant) did not include the liver.
Table 1: Pancreas transplant primary diagnosis, USA data (1994-
2020). Courtesy of Dr. Angelika Gruessner (IPTR), January 2021.
| Diagnosis |
PAK |
PTA |
SPK |
Total |
Diabetes secondary to chronic pancreatitis without
pancreatectomy |
1 |
4 |
9 |
14 |
Diabetes secondary to cystic fibrosis without
pancreatectomy |
2 |
1 |
3 |
6 |
| Pancreatic cancer |
0 |
2 |
2 |
2 |
| Bile duct cancer |
1 |
1 |
2 |
4 |
| Other cancer |
0 |
0 |
4 |
4 |
| Pancreatectomy prior to pancreas transplant |
1 |
41 |
10 |
52 |
| Diabetes mellitus- unknown etiology |
1 |
0 |
30 |
31 |
PAK: Pancreas after Kidney Transplant; PTA: Pancreas Transplant
Alone; SPK: Simultaneous Pancreas and Kidney Transplant.
Table 2: Pancreas transplants for cystic fibrosis, USA data (1988-
2020).
| Transplant type |
Frequency |
| PAK |
2 |
| PTA |
1 |
| SPK |
3 |
|
Liver Intestine Pancreas
|
2 |
| Liver Kidney Pancreas |
1 |
| Liver Pancreas |
14 |
| Liver Pancreas Lung |
2 |
| Pancreas Intestine |
1 |
PAK: Pancreas after Kidney Transplant; PTA: Pancreas Transplant
Alone; SPK: Simultaneous Pancreas and Kidney Transplant. There were 6
transplants in the traditional pancreas transplant categories and 20 vari-
ous multiorgan transplants including the pancreas.
Courtesy of Dr. Angelika Gruessner (IPTR), June 2021.
Usatin et al. [62] reviewed United Network for Organ Sharing
(UNOS) data from 1987-2014, and reported that of 4,600 patients
with cystic fibrosis, 17 patients underwent liver-pancreas, 4 patients pancreas-kidney, 3 patients pancreas-lung, 3 patients pancreas only, and 1 patient liver-lung transplants. Two-years graft
survival rates were 88% for liver-pancreas, 33% for lung-pancreas
and 100% for pancreas-kidney and pancreas alone transplants. It
was concluded that despite ninety percent of patients with cystic
fibrosis suffering from pancreatic exocrine insufficiency and 26%
developing diabetes after 10 years of the disease, pancreas transplant is still underutilized in these patients.
Other than chronic pancreatitis and cystic fibrosis, pancreas
transplants are performed in patients with benign pancreatic tumors such as intraductal papillary mucinous neoplasia (see below) [63]. There have been concerns regarding the use of immunosuppressive drugs in patients with a history of cancer. However,
currently, solid organ transplants are offered to patients after
being cancer-free for a certain period of time [64,65] or patients
with hepatocellular carcinoma [66-69] or colorectal cancer under
certain conditions [70,71]. One European study [63] showed that
when patients who had been cancer-free for a certain period of
time were accepted for pancreas transplantation, an increase of
15 pancreas transplants per year was noted in their program.
Cost effectiveness of pancreas allotransplantation for exocrine disorder
The cumulative cost of insulin for 20 years is estimated to be
about $663,000 per patient and 9.3 quality-adjusted life years.
The average cost-effectiveness ratio being $71,000 per quality-adjusted life years. The cumulative cost for islet allotransplantation
is estimated to be nadir of $519,000 and a cumulative effectiveness of 10.9 quality-adjusted life years [72]. Vrochides et al. [73]
showed that the cumulative cost for whole pancreas transplant is about $40,000. In 2014, a study from United Kingdom reported
12,000 admissions per year due to chronic pancreatitis. Estimated cost was £55.8 million per year. This is equal to £71,000 (or
$113,000) per patient per year [74]. Other reports [75-77] also
confirm the cost effectiveness of pancreas transplantation compared to other treatment options for chronic pancreatitis.
Historical overview of now obsolete pancreas auto- and allotransplants
For reasons of completeness, a history of now obsolete pancreas auto- and allotransplants is provided here as well [78].
Pancreas autotransplants for chronic pancreatitis
Pancreas autotransplants were basically performed at a time
when the islet isolation process in many ways was still in its infancy and islet cell yields were poor.
The concept of heterotopic autotransplantation of the segmental pancreas to treat chronic pancreatitis was introduced by Hogle
and Reemtsma in 1978 [79]. They described two cases in which
the segmental autografts were anastomosed with their splenic
vessels to the femoral vessels; the pancreatic ducts were ligated,
with one patient requiring drainage of a groin abscess. Of the two
patients, one had a functioning graft 3 years post-transplant; the
second was lost to follow-up. Tosarti et al. described three patients with chronic pancreatitis who underwent segmental autotransplants with vascular anastomosis also to the femoral vessels:
The pancreatic ducts were injected with 8 mL of neoprene, but
all three patients developed pancreatic fistulas [80]. At 12 to 16
months posttransplant, all three patients were free of pain and
insulin independent.
Rossi et al. described 10 patients with chronic pancreatitis who
underwent heterotopic segmental pancreatic autotransplants after near-total (95%) pancreatectomy: A small rim of pancreatic tissue was left attached to the duodenum to preserve the integrity
of the common bile duct and part of the duodenal blood supply
[81]. The pancreatic body and tail (50% to 60% of the gland) were
prepared for autotransplantation; the pancreatic duct was injected with 1.5 to 2.5 mL of neoprene and ligated. The remainder of
the resected pancreas was submitted for histopathologic studies.
The splenic vessels were anastomosed end to side to the common
femoral vessels, and the segmental autograft was placed in a subcutaneous pocket overlying the vastus lateralis muscle [81,82].
To reduce the risk of venous thrombosis, an arteriovenous fistula
between the distal splenic artery and vein was constructed in patients with small pancreas grafts. The initial bulge from the graft
progressively decreased and disappeared in 3 to 4 months. With
a median follow-up of 31 (range, 24 to 54) months, Rossi et al. reported that heterotopic pancreas autotransplants were technically successful in 8 of these 10 patients. Only one of them required
insulin at 2 years post-transplant; the other seven patients with
technically successful grafts had remained insulin independent.
Pancreas autotransplants provide an opportunity to assess the
long-term function of segmental grafts without the influence of
rejection and the effects of immunosuppression. In some series,
patients with near-total or staged-total pancreatectomy showed
decreased insulin responses after pancreas auto transplants
[81,82]. But, the loss of endocrine function as a result of ductal
occlusion occurred more slowly in humans than in large animals [83,84]. Hyperinsulinemia as a result of systemic vein drainage
has been documented after pancreas autotransplants. Rossi et al.
also found that patients with “idiopathic” chronic pancreatitis appeared to have better pain relief and better preservation of endocrine function, as compared with alcoholic patients with chronic
pancreatitis [82]. They also discussed the rationale for pancreas
(vs islet) auto transplants at the time. The combination of decreased islet cell mass, the low yield of then-current methods of
islet cell isolation, and the limited results reported with intraportal islet auto trans-plants for chronic pancreatitis had dissuaded
them from using islet auto transplants [82].
Subsequently, several modifications for heterotopic pancreas
auto transplants were reported: Use of the pancreatic body only,
with anastomosis of the proximal splenic vessels to the common
femoral vessels and ligation of both ends of the pancreatic duct
[85]; staged enteric drainage with a Roux-en-Y anastomosis to the
pancreatic duct [86]; extra peritoneal anastomosis of the splenic
vessels to the iliac vessels, with primary enteric drainage of the
pancreatic duct to a Roux-en-Y loop, with or without temporary
placement of a percutaneous stent in the pancreatic duct [87];
and extra peritoneal placement and anastomosis of the splenic
vessels to the iliac vessels with pancreaticocystostomy [88].
Auto transplants into the iliac fossa (with anastomosis to the
iliac vessels) appeared to be less prone to surgical complications
than autotransplants into the groin (with anastomosis to the
common femoral vessels). Groin complications, such as transient
or permanent pancreatic fistulas, pancreatitis, and hematomas
or bleeding from the femoral muscles, usually resulted in lower
quality of life, as compared with complications that arose from
the iliac fossa. As with segmental allotransplants, intraperitoneal
placement of segmental autotransplants appeared to cause the
lowest complication rate and so might be the best way to decrease posttransplant complications.
The surgical techniques for managing exocrine pancreatic secretions developed in a similar fashion for segmental autotransplants as they did for segmental allotransplants: from duct ligation and duct injection to enteric or bladder drainage. Although
improvements in exocrine function cannot be expected by enteric
drainage in patients with chronic pancreatitis, enteric drainage
may be the choice to preserve the existing level of exocrine function [85].
Removal of remaining ductal calculi and debris was desirable
but often not technically feasible. Yet, reestablishment of pancreatic duct patency may have prevented further progression of
fibrosis: Long-term evaluation of the exocrine pancreas function
(as assessed by the exocrine pancreas function diagnostic test,
expressed as the urinary excretion rate of orally administered paminobenzoic acid) showed that posttransplant values were either similar to or slightly higher than pretransplant values [87].
For those reasons, enteric drainage was considered the choice
for segmental autotransplants: Open duct drainage inevitably
caused pancreatic fistulas; duct ligation and duct occlusion may
have promoted progressive fibrosis of the pancreas graft, as in
large animals [83,84,89]; and bladder drainage may have required
bicarbonate supplementation in patients with remaining exocrine
function.
Preservation and storage of segmental autografts should be identical to those of segmental allografts from living or deceased
donors: Autografts should be flushed with small amounts (20 to
50 mL) of University of Wisconsin (UW) solution via the splenic
artery and, until implantation, stored in UW solution. To reduce
ischemia times of up to 300 minutes [84], the iliac vessels at the
implantation site should be dissected out before the splenic vessels of the native pancreas are ligated and divided. Decreased
ischemia time may benefit the remaining endocrine as well as
exocrine function of the segmental autograft.
Because of denervation of the autograft, autotransplants did
not appear to create the typical pain syndrome associated with
chronic pancreatitis. Although isolated occurrence of groin pain
and pancreatitis had been reported [81], it was most likely related
to the duct occlusion technique rather than to the underlying disease.
The primary aim of segmental pancreas autotransplants at the
time of insufficient islet processing was to preserve islet function
and prevent or delay the onset of diabetes mellitus. Short- and
long-term studies in recipients who were not insulin dependent
before their autotransplants showed that both oral and Intravenous Glucose Tolerance Tests (IVGTTs) in most remain similar
to, somewhat better than [87,88] or somewhat worse [82] than
their pretransplant state. In one recipient, only mild glucose intolerance was reported even 7 years after the autotransplant
[86]. But, in recipients who required insulin therapy before the
autotransplant, no improvements in glucose metabolism could be
expected. The question, then, was whether such patients should
undergo a transplant in the first place. However, at the time it
appeared that autotransplants may have helped some recipients
retain minimal insulin and glucagon function, resulting in a less
brittle form of diabetes mellitus than that of patients who underwent total pancreatectomy alone.
In 1990, Rossi et al. presented long-term results in 13 patients
who had undergone extensive pancreas resection and simultaneous segmental autotransplants (median follow-up, 62 months).
Of the 13 recipients, 11 had technically successful grafts: three
of 6 who underwent total pancreatectomy and 3 of 5 who underwent near-total resection remained insulin independent. Those
who required insulin required small doses and had stable diabetes. However, the rate of pain recurrence was higher in those
who underwent near-total resection and, for that reason, total
pancreatectomy as the initial procedure of choice was favored.
Rossi et al. concluded that total pancreatectomy and simultaneous segmental autotransplants offer definitive, although at times
transient, benefits in glucose metabolism, as compared with total
pancreatectomy alone [90].
In his last overview, published in 2003, on pancreas autotransplantation in patients with chronic pancreatitis, Rossi lists a total
of 28 such procedures: in 25 of them the femoral vessels and in
3 the iliac vessels were used for anastomosis; in 17 procedures,
the pancreatic duct was obliterated, in 11 ligated and in 1 entericdrained. There was 1 operative death; 5 patients developed pancreatic fistulae, 5 patients necroses, 3 patients abscesses. Remarkably, 16 of 28(57%) patients remained insulin-independent, 19 of
25(76%) were pain-free and in another 5(20%) the pain improved
[91]. If islet processing and yield improvement had not occurred
in the 1990s, pancreas autotransplantation may still be around.
Pancreas autotransplants for malignancy
Pancreas autotransplants even for small pancreatic malignancy
are no longer performed. If an auto-transplant is performed at all
at an early pancreatic tumor stage, it would be an islet autotransplant.
In the past, heterotopic segmental autotransplants after total
pancreatectomy were reported in few patients with periampullary cancer or advanced gastric cancer. In 1983, McDonald et
al. described a 73-year-old patient with a movable mass (3 to 5
cm) in the pancreatic head. At the time of resection, no positive
lymph nodes were noted and the distal pancreas was tumor free.
The distal pancreas was removed in vivo, the pancreatic duct was
ligated, and the distal pancreas was autografted into the thigh.
Tumor recurrence was not reported, but follow-up time was only
8 months [92]. In a second patient with advanced periampullary
cancer, the segmental pancreas autograft was also anastomosed
to the left femoral vessels. On completion of total pancreatectomy, the distal pancreas was noted to be free from cancerous
invasion. The pancreas was transected at the pancreatic body, 3
cm away from the tumor; cold ischemia time was 105 minutes.
Posttransplant, insulin requirements decreased and insulin administration was discontinued at 5 months [93].
Tersigni et al. described three irradiated segmental pancreas
autotransplants in patients with cancer of the pancreatic head
[94]. The autografts with ligated ducts were irradiated with 2,000
to 5,000 rad, doses believed to not affect β- and α-cells. After irradiation, the tumor-free distal segmental pancreases were autotransplanted by anastomosing the splenic vessels to the common
femoral vessels. The first graft (5,000 rad) became necrotic and
was removed 2 weeks posttransplant; the second and third autografts (2,000 rad each) were functioning and the patients were
insulin independent at 7 months and 1 month posttransplant, respectively. High-dose irradiation was used to (1) completely destroy any remaining multicentric tumor foci in the distal pancreas
and (2) decrease exocrine secretions. However, the two patients
with functioning autografts subsequently developed abdominal
metastases and, after beginning chemotherapy, had to resume
insulin. Despite irradiation, denervation, and the heterotopic location of the autograft, both patients’ intial plasma, insulin, and
glucagon levels were within normal range; responses to oral GTTs
and IV arginine stimulation tests were normal [95].
In another series, nine patients with advanced gastric cancer
underwent total gastrectomy, total pancreatectomy, and simultaneous segmental pancreas autotransplants with anastomosis of
the splenic vessels to either the external iliac or common femoral
vessels. Pancreatic exocrine secretions were managed by external, enteric, or bladder drainage. A total of four grafts were lost
because of surgical complications (venous thrombosis, leakage),
but five recipients remained insulin independent (follow-up, 7 to
41 months) [88].
On another historical note, in 1970 Urea et al. described allotransplanting a pancreatic insulinoma into the thigh of an insulin-resistant patient with juvenile diabetes mellitus. Although the
17-year-old recipient was aglycosuric for 47 days, insulin independence in the absence of immunosuppression was never achieved
[96] It is obvious from the above (anecdotal) reports with short
follow-up that segmental pancreas autotransplants in patients undergoing total pancreatectomy for malignancy were extremely
rare even in the pre-islet autotransplant era. The possible presence of occult pancreatic cancer cells in all types of pancreatic
autografts (segments or islets) is a major concern.
This controversial issue is confirmed by the Milan group in a
2024 article that reported their experience in 75 patients with
malignant pancreatic neoplasms who underwent an islet autotransplant after total or subtotal pancreatectomy. On follow-up,
they noted metastatic liver and lung disease in 17 (23%) patients
[99]. Further insights must be gained before even islet autotransplantation can be recommended routinely for patients undergoing resection of a pancreatic malignancy.
Pancreas transplants as part of cluster transplants for upperabdominal malignancies
In 1989, Starzl et al. reported on abdominal organ cluster
transplants for the treatment of upper-abdominal malignancies
[97]. However, long-term outcome was poor due to a high cancer recurrence rate. Starzl summarized it best by stating that “the
marriage of transplantation and therapeutic oncology has been
troubled” [97] -”troubled” both by the necessity of administering immunosuppressive therapy and by the natural behavior of
upper-abdominal malignancies.
The field of pancreas transplantation after total pancreatectomy continues to evolve. Generette et al. in 2020 reported on a
case of en-bloc liver and pancreas allotransplantation after total
pancreatectomy with autologous islet transplantation. The patient with intractable and debilitating pain secondary to chronic
pancreatitis had initially undergone a TPIAT. Subsequently, the
patient developed alcohol related acute liver failure and en-bloc
liver and pancreas transplantation was performed to replace the
failing liver with engrafted islets. A successful pancreas transplantation was performed to resolve his life-threatening severe hypoglycemic episodes [98].
References
- Laftavi RM, Pankewycz O, Gruessner RWG. Treatment of Pancreatic Exocrine Disorders by Pancreas and Islet Transplantation. In: Transplantation of the Pancreas. 2nd Edition. Editors: Gruessner RWG, Gruessner AC. Cham: Springer International Publishing. 2023; 80: 1101-1112.
- Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016; 16: 218-224.
- Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality of life in chronic pancreatitis: a case-control study from the North American Pancreatitis Study 2 cohort. Pancreas. 2013; 42: 293-300.
- Anderson MA, Akshintala V, Albers KM, et al. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology. 2016; 16: 83-94.
- Machicado JD, Amann ST, Anderson MA, et al. Quality of life in chronic pancreatitis is determined by constant pain, disability/unemployment, current smoking, and associated co-morbidities. Am J Gastroenterol. 2017; 112: 633-642.
- Rickels MR, Bellin M, Toledo FG, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: Recommendations from Pancreas Fest 2012. Pancreatology. 2013; 13: 336-342.
- Kleeff J, Whitcomb DC, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017; 3: 17060.
- Bellin MD, Whitcomb DC, Abberbock J, et al. Patient and disease characteristics associated with the presence of diabetes mellitus in adults with chronic pancreatitis in the United States. Am J Gastroenterol. 2017; 112: 1457-1465.
- Kloppel G, Maillet B. Pathology of acute and chronic pancreatitis. Pancreas 1993; 8: 659–70.
- Boerma D, Straatsburg IH, off erhaus GJ, Gouma DJ, van Gulik TM. Experimental model of obstructive, chronic pancreatitis in pigs.Dig Surg. 2003; 20: 520-26.
- Madsen P, Winkler K. The intraductal pancreatic pressure in chronic obstructive pancreatitis. Scand J Gastroenterol. 1982; 17: 553-54.
- Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015; 149: 39-51.
- Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015; 385: 1460-71.
- Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. 2013; 62: 1771-76.
- www.pancreasfoundation.org. 2020.
- Muniraj T, Aslanian HR, Farrell J, et al. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014; 60(12): 530-50.
- Aoun E, Chang CC, Greer JB, Papachristou GI, Barmada MM, et al. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS One 2008; 3: e2003.
- Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013; 144(6): 1282-91.
- Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. 2007; 56: 534-44.
- Bockman DE, Buchler M, Malfertheiner P, et al. Analysis of nerves in chronic pancreatitis. Gastroenterology. 1988; 94: 1459-69.
- Keith RG, Keshavjee SH, Kerenyi NR. Neuropathology of chronic pancreatitis in humans. Can J Surg. 1985; 28: 207-11.
- Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain-a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009; 136: 177-86. e1.
- Di Sebastiano P, Fink T, Weihe E, et al. Immune cell infiltration and growth associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology. 1997; 112: 1648-55.
- Ceyhan GO, Deucker S, Demir IE, et al. Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Lab Invest. 2009; 89: 347-61.
- Winstead n, Wilcox C. Clinical Trials of pancreatic enzyme replacement for painful chronic pancreatitis. A review. Pancreatology. 2009; 9: 344-350.
- Ajith K Siriwardena, James M Mason, Aali J Sheen, Alistair J Makin, Nehal S Shah. Antioxidant Therapy Does Not Reduce Pain in Patients with Chronic Pancreatitis: The ANTICIPATE Study. Gastroenterology. 2012; 143(3): 655-663.
- Cuschieri A, Shimi SM, Crosthwaite G, Joypaul V. Bilateral endoscopic splanchnicectomy through a posterior thoracoscopic approach. J R Coll Surg Edinb. 1994; 39: 44-47.
- Buscher HC, Schipper EE, Wilder-Smith OH, Jansen JB, vanGoor H. Limited effect of thoracoscopic splanchnicectomy in the treatment of severe chronic pancreatitis pain: a prospective long-term analysis of 75 cases. Surgery. 2008; 143: 715-722.
- Howard TJ, Swofford JB, Wagner DL, Sherman S, Lehman GA. Quality of life after bilateral thoracoscopic splanchnicectomy: Longterm evaluation in patients with chronic pancreatitis. J Gastrointest Surg. 2002; 6: 845-852.
- Drewes AM, Krarup AL, Detlefsen S, Malmstrom ML, Dimcevski G, et al. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. 2008; 57: 1616-1627.
- Buscher HC, van Goor H, Wilder-Smith OH. Effect of thoracoscopic splanchnic denervation on pain processing in chronic pancreatitis patients. Eur J Pain. 2007; 11: 437-443.
- Baghdadi S, Abbas MH, Albouz F, Ammori BJ. Systematic review of the role of thoracoscopic splanchnicectomy in palliating the pain of patients with chronic pancreatitis. Surg Endosc. 2008; 22: 580-588.
- Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack astroenterol. 2012; 107: 1096-103.
- Du Val MK Jr. Caudal pancreaticojejunostomy for chronic relapsing pancreatitis. Ann Surg. 1954; 140: 775.
- Zollinger RM, Keith LM, Ellison EH. Pancreatitis. N Engl J Med. 1954; 251: 497-502.
- Puestow CB, Gillesby WJ, et al. Retrograde surgical drainage of pancreas for chronic relapsing pancreatitis. Arch Surg. 1958; 76: 898-906.
- Partington PF, Rochelle REL. Modified Puestow procedure for retrograde drainage of the pancreatic duct. Ann Surg. 1960; 152: 1037-1043.
- Frey CF, Smith GJ. Description and rationale of a new operation for chronic pancreatitis. Pancreas. 1987; 2: 701-707.
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935; 102: 763-779.
- Beger HG, Witte С, Kraas E, et al. Erfahrung mit einer das duodenum erhaltenden pankreaskopfresektion bei chronischer Pankreatitis. Chirurg. 1980; 51: 303-307.
- Easter DW, Cuschieri A. Total pancreatectomy with preservation of the duodenum and pylorus for chronic pancreatitis. Ann Surg. 1991; 214: 575-580.
- Fleming WR, Williamson RCN. Role of total pancreatectomy in the treatment of patients with end-stage chronic pancreatitis. Br J Surg. 1995; 82: 1409.
- Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1998; 207: 39-47.
- Russell RCG. Total pancreatoduodenectomy for chronic pancreatitis. In: Trede M, Carter DC, eds. Surgery of the Pancreas. London: Churchill-Livingstone. 1997: 369-378.
- McCullagh EP, Cook JR, Shirley EK. Diabetes following total pancreatectomy: Clinical observations of ten cases. Diabetes. 1958; 7: 298-307.
- Robertson RP, Lanz KJ, Sutherland DER, et al. Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes. 2001; 50: 47-50.
- Büchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Archives of Surgery. 2003; 12: 1310-1314.
- Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Annals of Surgery. 2007; 6: 966-975.
- Billings BJ, Christein JD, Harmsen WS, et al. Quality-of-life after total pancreatectomy: Is it really that bad on long-term follow-up? Journal of Gastrointestinal Surgery. 2005; 8: 1059-1067.
- Belyaev O, Herzog T, Chromik AM, et al. Early and late postoperative changes in the quality of life after pancreatic surgery.
- Robert Moran, Robert Klapheke, George K John, Sarah Devlin, et al. Prevalence and predictors of pain and opioid analgesic use following total pancreatectomy with islet autotransplantation for pancreatitis Pancreatology. 2017; 17(5): 732-737.
- Dresler CM, Fortner JG, Mcdermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991; 214(2): 131‐140.
- Andersen DK, Andren‐sandberg Å, Duell EJ, et al. Pancreatitis‐di‐abetes‐pancreatic cancer: summary of an NIDDK‐NCI workshop. Pancreas. 2013; 42(8): 1227‐1237.
- Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from Pancreas Fest. Pancreatology. 2014; 14(1): 27‐35.
- Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005; 201(5): 680‐687.
- David Adams, Chung Catherine, Owczarski Stefanie, Wang Hongjun,Morgan Katherine: Long-Term Survival after Total Pancreatectomy with Islet-Auto-Transplantation (TPIAT) for Chronic Pancreatitis Pancreatology. 2017; 17: 74.
- Al‐sofiani ME, Quartuccio M, Hall E, Kalyani RR. Glycemic outcomes of islet autotransplantation. Curr Diab Rep. 2018; 18(11): 116.
- Laftavi MR, AC, Gruessner R. Surgery of pancreas transplantation. Curr. Opin. Transp. 2017; 22(4): 389-397.
- Gruessner A, Gruessner RW: Long-term outcome after pancreas transplantation: A registry analysis. Curr Opin Organ Transplant. 2016; 21(4): 377-85.
- Gruessner RW, Manivel C, Dunn DL, Sutherland DE. Pancreaticoduodenal transplantation with enteric drainage following native total pancreatectomy for chronic pancreatitis: A case report. Pancreas. 1991; 6(4): 479‐488.
- Gruessner RW, Sutherland DE, Drangstveit MB, Kandaswamy R, Gruessner AC. Pancreas allotransplants in patients with a previous total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2008; 206(3): 458‐465.
- Usatin DJ, Perito ER, Posselt AM, Rosenthal P. Under utilization of pancreas transplant in cystic fibrosis recipients in the United Network Organ Sharing (UNOS) data 1987-2014. Am. J. of Transplantation. 2016; 16: 1620-1625.
- Mehrabi A, Golriz M, Adili-Aghdam F, et al. Expanding the Indications of Pancreas Transplantation Alone. Pancreas. 2014; 43(8): 1190-1193.
- Cheung CY, Tang SCW. An update on cancer after kidney transplantation. Nephrol Dial Transplant. 2019; 34(6): 914-920.
- Pendón-Ruiz de Mier MV, Agüera ML, Navarro MD, Rodriguez-Benot A. Aljama: Prevalence and Survival of Cancer After PancreasKidney Transplantation.Transplant Proc. 2018; 50(2): 669-672.
- Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018; 67(1): 381-400.
- Yadav DK, Chen W, Bai X, Singh A, Li G, Ma T, Yu X, Xiao Z, Huang B, Liang T: Salvage Liver Transplant versus Primary Liver Transplant for Patients with Hepatocellular Carcinoma. Ann Transplant. 2018; 23: 524-545.
- Gunsar F: Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria.Exp Clin Transplant. 2017; 15(Suppl 2): 59-64.
- Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013; 257(5): 800-6.
- Simoneau E, D’Angelica M, Halazun KJ. Liver transplantation for colorectal liver metastasis. Curr Opin Organ Transplant. 2019; 24(2): 175-181.
- Gorgen A, Muaddi H, Zhang W, McGilvray I, Gallinger S, et al. The New Era of Transplant Oncology: Liver Transplantation for Nonresectable Colorectal Cancer Liver Metastases. Can J Gastroenterol Hepatol. 2018; 2018: 9531925.
- Beckwith J, Nyman JA, Flanagan B, et al. A health economic analysis of clinical islet transplantation. Clin Transplant. 2012; 26: 23-33.
- Vrochides D, Paraskevas S, Papanikolaou V. Transplantation for type 1 diabetes mellitus. Whole organ or islets? Hippokratia. 2009; 13: 6-8.
- Hall TC, Garcea G, Webb MB, et al. The socio-economic impact of chronic pancreatitis: A systematic review. Journal of Evaluation in Clinical Practice. 2014; 20: 203-207.
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343: 230-238.
- Frank A, Deng S, Huang X, et al Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004; 240: 640-643.
- Demartines N, Schiesser M, Clavien PA. An evidence-based analysis of simultaneous pancreas-kidney and pancreas transplantation alone. Am J Transplant. 2005; 5: 2688-2697.
- Gruessner RWG: Treatment of pancreatic exocrine disorders by pancreas and islet transplantation. In: Pancreas Transplantation. Gruessner RWG and Sutherland DER (editors). Springer, Inc, New York, 1st edition. 2004; 22: 627-637.
- Hogle HH, Reemtsma K. Pancreatic autotransplantation following resection. Surgery. 1978; 82: 359-360.
- Tosatti E, Valente U, Campisi С, et al. Segmental pancreas autotransplantation in man following total or near total pancreatectomy for serious recurrent chronic pancreatitis. Transplant Proc. 1980; 12: 15-18.
- Rossi RL, Braasch JW, Nugent FW, et al. Segmental pancreatic autotransplantation for chronic pancreatitis. Surgery. 1983; 145: 437-442.
- Rossi RL, Soeldner JS, Braasch JW, et al. Segmental pancreatic autotransplantation with pancreatic ductal occlusion after near total or total pancreatic resection for chronic pancreatitis. Results at 5- to 54-month follow-up evaluation. Ann Surg. 1986; 203: 626-636.
- Garvin PJ, Castaneda M, Codd JE, et al. A comparison of ductal management techniques in an in situ canine pancreas model. Arch Surg. 1984; 119: 829-832.
- Nghiem DD, Pitzen RH, Corry RJ. Evaluation of techniques of controlling exocrine drainage after segmental pancreatectomy in dogs. Arch Surg. 1985; 120: 1132-1133.
- Miyata M, Nakao K, Izukuru M, et al. Segmental auto-transplantation of the pancreas. Jpn J Surg. 1987; 17: 41-46.
- Soon-Shiong P, Swafford G, Levin S. Successful long-term exocrine and endocrine function of the autotransplanted pancreas in humans. Pancreas. 1987; 2: 357-361.
- Tamura K, Yano S, Kin S, et al. Heterotopic autotransplantation of a pancreas segment with enteric drainage after total or subtotal pancreatectomy for chronic pancreatitis. Int J Pancreatol. 1993; 13: 119-127.
- Fukushima W, Shimizu R, Isumi R, et al. Heterotopic segmental pancreatic autotransplantation in patients undergoing total pancreatectomy. Transplant Proc. 1994; 26: 2285-2287.
- Gooszen HG, Bosman FT, vanSchilfgaarde R. The effect of duct obliteration on the histology and endocrine function of the canine pancreas. Transplantation. 1984; 38: 13-17.
- Rossi RL, Soeldner JS, Braasch JW, et al. Long-term results of pancreatic resection and segmental pancreatic autotransplantation for chronic pancreatitis. Am J Surg. 1990; 159: 51-58.
- Watkins JG, Krebs A, Rossi RL. Pancreatic autotransplantation in chronic pancreatitis. World J Surg. 2003; 27(11): 1235-40.
- McDonald JC, Rohr MS, Tucker WY. Recent experiences with autotransplantation of the kidney, jejunum, and pancreas. Ann Surg. 1983; 197: 678-686.
- Mitsuno M, Miyata M, Okuda A, et al. Segmental autotransplantation of the pancreas after total pancreatectomy for advanced periampullary carcinoma-a case report. Jpn J Surg. 1988; 18: 363-368.
- Tersigni R, Toledo-Pereyra LH, Fallucca F, et al. Transplantation of irradiated heterotopic segmental human pancreas. Am Surg. 1983; 49: 502-505.
- Fallucca F, Tersigni R, Giangrande L, et al. Insulin, C-peptide, glucagon, and somatostatin secretion in segmental pancreatic autotransplantation. Transplant Proc. 1984; 16: 741-744.
- Urea I, Kott I, Lev-Ran A, et al. Transplantation of a pancreatic insulinoma into an insulin-resistant patient with juvenile diabetes. Diabetes. 1970; 19: 182-185.
- Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989; 210: 374-386.
- Generette G, Bachul P, Golab K, Baso L, et al. En bloc liver and pancreas transplantation after total pancreatectomy with autologous islet transplantation. Eur J Transl Clin Med. 2020; 3(2): 11-17.
- Piemonti L, Melzi R, Aleotti F, et al. Autologous Pancreatic Islet Cell Transplantation Following Pancreatectomy for Pancreas Diseases Other Than Chronic Pancreatitis: A 15-Y Study of the Milan Protocol. Transplantation. 2024: 10-97.