Introduction
Peritoneal mesothelioma, a rare and aggressive cancer originating from the mesothelial lining of the peritoneum, represents
a challenging clinical entity. Despite its infrequency, accounting
for only 15% of all mesothelioma cases, it poses significant diagnostic and therapeutic dilemmas [1]. Here, we provide a comprehensive overview of the current understanding of peritoneal mesothelioma, encompassing epidemiology, diagnostic modalities,
histopathological classification, molecular markers, and contemporary therapeutic strategies.
Case presentation
We present a 45-year-old male with a history for peptic ulcer
disease and alcohol use disorder, now abstinent for one year.
He presented to the emergency department with progressively
worsening abdominal pain and constipation, accompanied by
symptoms of anorexia and weight loss. On physical examination,
abdominal distention and diffuse tenderness to palpation were
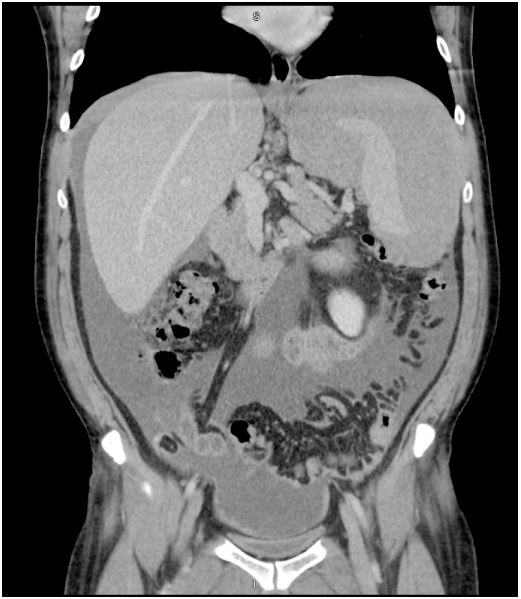
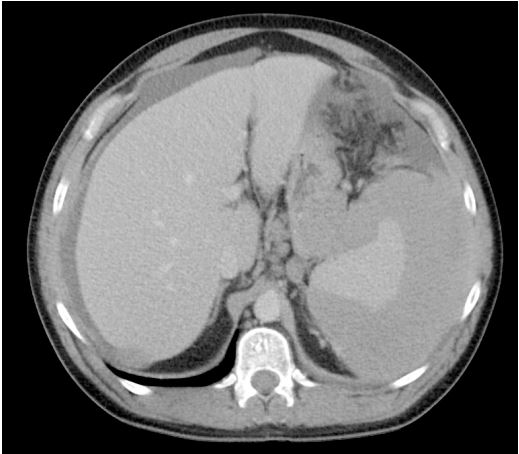
noted. CT abdomen with contrast (Figures 1 and 2) revealed a
large, complex fluid collection or irregular soft tissue surrounding
the spleen, along with thickening of the right hemidiaphragm and
adjacent liver. Additionally, there was mild ascites with associated
stranding and edema of the anterior mesentery. A peri splenic
mass biopsy demonstrated an epithelioid neoplasm with mild to
moderate atypia. Immunohistochemistry was positive for CK7,
D2-40, and calretinin, while negative for TTF-1, arginase, glypican-3, AFP, CDX2, CK20, HepPar 1, consistent with an epithelioid
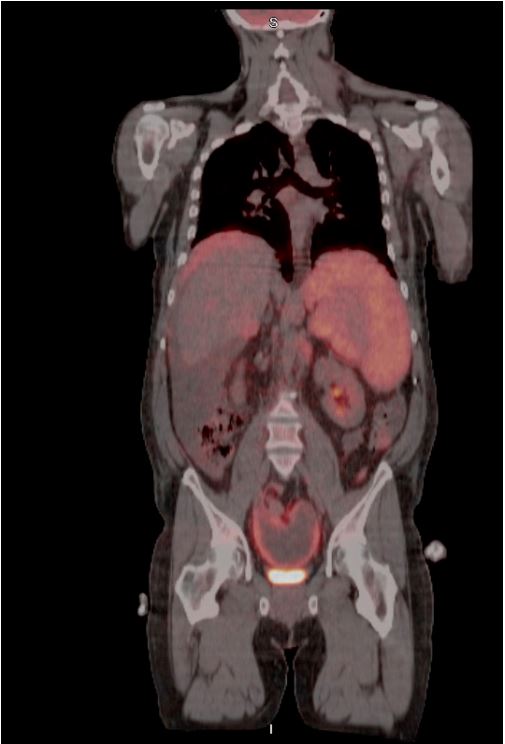
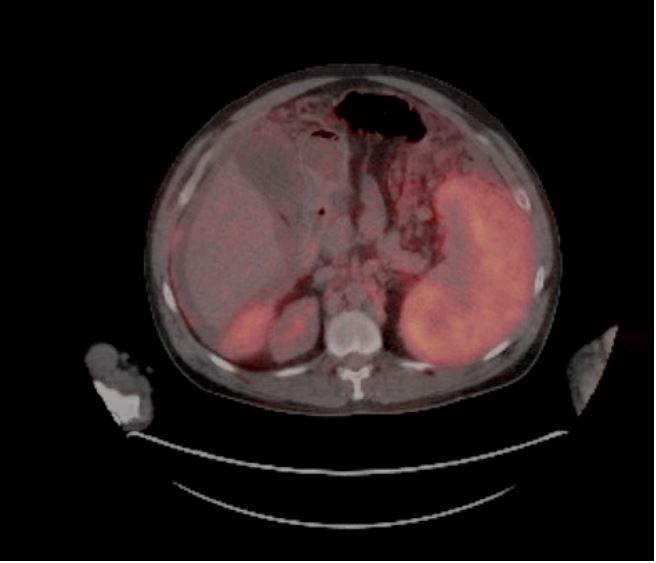
mesothelioma. A PET/CT scan (Figures 3 and 4) revealed FDG-avid
masses involving the peri splenic, perihepatic, and pelvic peritoneum, the left lower pelvis and around the falciform ligament,
with suspicion for liver involvement as well as omental caking in
the upper and mid-abdominal regions. Additionally, mildly FDGavid lymphadenopathy was observed, raising concern for metastatic disease. Upon multidisciplinary discussion, the consensus
was to proceed with cervical/axillary lymph node biopsy to confirm metastatic disease. Depending on the biopsy results, surgical debulking with Hyperthermic Intraperitoneal Chemotherapy
(HIPEC) would be considered if the disease was non-metastatic,
whereas palliative chemotherapy with cisplatin and pemetrexed
would be proposed if lymph node metastasis was confirmed.
Methods
Literature review: A comprehensive literature search was conducted using electronic databases including PubMed, Embase,
and Google Scholar. The search strategy employed a combination
of Medical Subject Headings (MeSH) terms and keywords related
to peritoneal mesothelioma, epidemiology, diagnostic modalities,
histopathological classification, molecular markers, and therapeutic strategies. Articles published in English from inception to the
present were considered for inclusion. The search was augmented by manual screening of reference lists from relevant articles to
ensure comprehensive coverage of the topic.
Epidemiology and clinical presentation
Peritoneal mesothelioma demonstrates an annual incidence
of approximately 1.2 per million person-years in men and 0.8
per million person-years in women [2]. Although accounting for
a minority of mesothelioma cases, its clinical manifestations are
often nonspecific, including abdominal pain, palpable mass, and
ascites, leading to delayed diagnosis [3]. Notably, peritoneal mesothelioma shares histopathological features with its more common pleural counterpart, influencing diagnostic and therapeutic
approaches.
Diagnostic modalities
The diagnosis of peritoneal mesothelioma relies on a combination of imaging techniques and histopathological evaluation.
Serum markers, such as CA-125 and Soluble Mesothelin-Related
Peptide (SMRP) can be supportive. Contrast-enhanced CT of the
chest, abdomen, and pelvis, as well as FDG-PET/CT scan, play pivotal roles in delineating disease extent and guiding biopsy. Laparoscopic or image-guided biopsy remains the gold standard for
histological confirmation, with immunohistochemistry providing
valuable insights into tumor subtyping [4].
Histopathological and molecular characterization
Peritoneal mesothelioma encompasses three histological subtypes: epithelioid, sarcomatoid, and biphasic, each bearing distinct prognostic implications. Immunohistochemistry, utilizing
markers such as calretinin and D2-40, aids in subtype classification
[5]. Furthermore, emerging evidence highlights the relevance of
cytogenetic, including CDKN2A deletion and BAP1 mutation, and
molecular markers EWSR1:ATF1 fusion and ALK rearrangements
in refining diagnostic accuracy and prognostication [6,7]. Notably,
germline mutations such as BAP1, BRCA2, CDKN2A, TMEM127,
VHL, WT1, MRE11A, and MSH6 are identified in 12-16% of mesotheliomas, particularly in young individuals with a family history
of mesothelioma or synchronous malignancies [8].
Therapeutic strategies
The cornerstone of treatment for peritoneal mesothelioma is
Cytoreductive Surgery (CRS) combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) [9]. CRS aims to debulk tumors
maximally, followed by intraperitoneal administration of heated
chemotherapeutic agents, including cisplatin, doxorubicin, carboplatin, and mitomycin C. Patient selection for CRS+HIPEC depends
on histological subtype, disease burden, and performance status,
with meticulous attention to perioperative management and surveillance.
This treatment strategy is tailored to individual tumor characteristics and patient factors. It is primarily indicated for epithelioid
tumors with favorable prognostic factors (e.g., medically operable
with Complete Cytoreduction (CC) score i.e. CC-0 or CC-1 [10], absence of lymph node involvement, Ki-67 ≤9%, Peritoneal Cancer
Index [PCI] ≤17) [11]. Biphasic/sarcomatoid tumors may undergo
CRS+HIPEC after tumor regression with systemic chemotherapy
or in cases of recurrent disease after previous CRS beyond 12
months [12].
A vigilant surveillance protocol is recommended for patients
with favorable prognostic profiles, including regular imaging with
CT of the chest, abdomen, and pelvis every 3-6 months for 5
years, followed by yearly follow-up until 10 years post-treatment.
Conversely, tumors with high-risk features, such as elevated Ki-67,
nodal metastasis, high tumor burden (PCI>17), or biphasic disease,
may require systemic therapy before considering CRS+HIPEC.
Systemic therapy options encompass various chemotherapeutic agents, such as cisplatin, pemetrexed [13], and bevacizumab
[14], along with immunotherapy using agents like nivolumab and
ipilimumab [15]. Additional agents like gemcitabine and vinorelbine [16] may be utilized in specific clinical scenarios. Patients
experiencing tumor progression following initial treatment may
benefit from pemetrexed-based regimens or immunotherapy
[17], guided by comprehensive molecular profiling to identify potential targetable mutations.
For patients with poor performance status (ECOG PS 3-4), supportive care measures are crucial for symptom management and
palliation. These include interventions like paracentesis for ascites, symptom control for nausea, vomiting, and pain, as well as access to palliative care services aimed at optimizing quality of life.
Future directions
The advent of targeted therapies and immunomodulatory
agents heralds a paradigm shift in the management of peritoneal
mesothelioma. Comprehensive molecular profiling holds promise in identifying actionable genetic alterations and personalizing
therapeutic interventions. Moreover, ongoing clinical trials investigating novel treatment modalities underscore the imperative of
multidisciplinary collaboration and translational research in optimizing patient outcomes.
Conclusion
Peritoneal mesothelioma poses formidable challenges to clinicians and researchers alike, necessitating a nuanced understanding of its epidemiology, pathogenesis, and therapeutic landscape.
Through concerted efforts in advancing diagnostic precision and
therapeutic efficacy, we strive towards improving the prognosis
and quality of life for affected individuals.
References
- Tsao MS, Nicholson AG, Maleszewski JJ, Marx A, Travis WD. Introduction to 2021 WHO Classification of Thoracic Tumors. Journal of Thoracic Oncology. 2022; 17(1): e1-4.
- Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009; 20(6): 935-44.
- Malpica A. Peritoneal Mesothelioma-An Update. Advances in Anatomic Pathology. 2023; 30(4): 262.
- Laterza B, Kusamura S, Baratti D, Oliva GD, Deraco M. Role of explorative laparoscopy to evaluate optimal candidates for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal mesothelioma. In Vivo. 2009; 23(1): 187-90.
- Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018; 142(1): 89-108.
- Churg A, Naso JR. The Separation of Benign and Malignant Mesothelial Proliferations: New Markers and How to Use Them. Am J Surg Pathol. 2020; 44(11): e100-12.
- Identification of ALK Rearrangements in Malignant Peritoneal Mesothelioma | Genetics and Genomics | JAMA Oncology | JAMA Network. https://jamanetwork.com/journals/jamaoncology/fullarticle/2653897
- Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, et al. A Subset of Mesotheliomas with Improved Survival Occurring in Carriers of BAP1 and Other Germline Mutations. J Clin Oncol. 2018; 36(35): JCO2018790352.
- Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009; 27(36): 6237-42.
- Votanopoulos KI, Sugarbaker P, Deraco M, Morris D, Glehen O, et al. Is Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy Justified for Biphasic Variants of Peritoneal Mesothelioma? Outcomes from the Peritoneal Surface Oncology Group International Registry. Ann Surg Oncol. 2018; 25(3): 667-73.
- Gilly FN, Cotte E, Brigand C, Monneuse O, Beaujard AC, Freyer G, et al. Quantitative prognostic indices in peritoneal carcinomatosis. Eur J Surg Oncol. 2006; 32(6): 597-601.
- Pasqual EM, Londero AP, Robella M, Tonello M, Sommariva A, et al. Repeated Cytoreduction Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Selected Patients Affected by Peritoneal Metastases: Italian PSM Oncoteam Evidence. Cancers (Basel). 2023; 15(3): 607.
- Carteni G, Manegold C, Garcia GM, Siena S, Zielinski CC, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009; 64(2): 211-8.
- Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet. 2016; 387(10026): 1405-14.
- Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, et al. Firstline nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet. 2021; 397(10272): 375-86.
- Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): A multicentre randomised trial - PubMed. 2024. https://pubmed.ncbi.nlm.nih.gov/18486741/
- Second-line chemotherapy in malignant pleural mesothelioma: Results of a retrospective multicenter survey - PubMed. 2024. https://pubmed.ncbi.nlm.nih.gov/21937142/.